Lenacil Is An Active Ingredient for Plant Protection
General Description

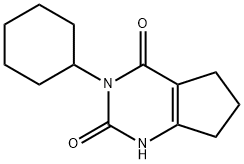
Figure 1. Properties of Lenacil
Lenacil (Herbicide 634), is the ISO common name for 3-cyclohexyl-1,5,6,7-tetrahydrocyclopentapyrimidine-2,4(3H)-dione (IUPAC). It is an odorless, white crystalline compound that is soluble and stable in water, aqueous acids and common organic solvents but is subject to microbial decomposition under moist conditions in soil: it is the active component of Venzar@ lenacil herbicide (a wettable powder containing 80 % w/w lenacil), employed for selective weed control in sugar beet crops (du Pont de Nemours, 1967). Pease (1966) described a gas chromatography method for the determination of lenacil residues in this crop and in soil. 1,2
Usage
Lenacil is used as an active ingredient in crop protection products. It is a selective pre-emergence herbicide that is absorbed through the roots and acts by inhibiting photosynthesis. It is mainly used against seed weeds and annual grasses in spinach, sugar beets, strawberries and ornamental plants. It is distributed by DuPont.3
Influence of lenacil on rye seedling biomass
The fresh weight of green rye seedlings was generally decreased by lenacil. Only at the highest temperature its action turned out to be not significant compared to control plants. Action of lenacil on dry biomass was less consistent; this chemical reduced weights of both green seedlings grown at 15 °C and etiolated plant at 22 °C. In turn, biomass of light-grown plants in the presence of lenacil increased only at the highest temperature. Under remained conditions changes caused by lenacil were considered not significant compared with respective controls. Application of lenacil markedly decreased the percentage of moisture in rye seedlings grown at 29 and 15 °C, independent of light. Treatment with lenacil usually impaired growth of rye seedlings. The impact of the others on this cereal growth was markedly dependent on temperature. Lenacil also stimulated the biosynthesis of resorcinolic lipids in green rye seedlings grown at 22 °C. Compared to respective controls, these herbicides caused an increase by 184% and 52%, respectively. Under dark conditions their influences were opposite and resulted in decreased levels of ARs. Linuron tested at this temperature decreased the alkylresorcinol content by 23% and 35% in green and etiolated plants, respectively.4
Safety
The manufacturers had reported that maximal feasible oral doses of 11 g/kg in the rat, and percutaneous doses of 5 g/kg in the rabbit, did not produce mortality, clinical evidence of toxicity or gross pathological changes. Lenacil was found to have little evidence of toxicity, and its effect upon food utilization and rate of body weight gain of rats in the long-term study was not manifested until the daily intake had risen to approximately 400 mg/kg in females and 800 mg/kg in males, at which levels the possibility of some interference with nutrition or with the alimentary flora cannot be excluded. Increased relative liver weight at these levels may similarly be regarded as without toxicologic significance in the absence of other evidence of liver damage (Golberg, 1966). This almost complete freedom from demonstrable effects was commensurate with the lack of mortality and signs of toxicity in the acute tests reported by the manufacturer. The rat reproductive studies did not reveal any adverse effect, or indication of teratogenicity or mutagenicity. The dog study provides even less evidence of toxicity, and the accidental production of healthy offspring by 2 of the females receiving 10,000 ppm lenacil in the diet suggested that lenacil was probably without adverse effect upon female reproductive performance in this species.5
Toxicity Data
LD50 (80% wettable powder) orally in rats: >11,000 mg/kg; LC50 96 hr in bluegill sunfish; rainbow trout: 100-1000; 135 mg/l; LC50 8 day in bobwhite quail, Peking duck (mg/kg): 2300, >5620. (DuPont technical data sheet)6
Synthesis
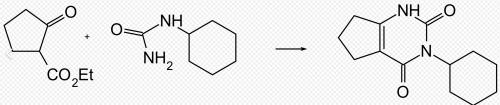
Figure 2. Synthesis of lenacil
Lenacil can be obtained by a condensation reaction of ethyl 2-oxocyclopentane-l-carboxylate and cyclohexylurea with phosphoric acid.7
References
1.M. Bahadir, H. Parlar, Michael Spiteller: Springer Environmental Lexicon . Springer, 2000, ISBN 978-3-540-63561-1.
2.Ullmann's Agrochemicals, Volume 1 . Wiley-VCH, 2007, ISBN 978-3-527-31604-5.
3.T. R. Roberts, DH Hutson: Metabolic pathways of agrochemicals, Volume 2. Royal Soc of Chemistry, 1999, ISBN 978-0-85404-499-3.
4.Magnucka, E. G., Pietr, S. J., Kozubek, A., & Zarnowski, R. (2014). Various effects of the photosystem II–inhibiting herbicides on 5-n-alkylresorcinol accumulation in rye seedlings. Pesticide biochemistry and physiology, 116, 56-62.
5.Worden, A. N., Noel, P. R., Mawdesley-Thomas, L. E., Palmer, A. K., & Fletcher, M. A. (1974). Feeding studies on lenacil in the rat and dog. Toxicology and applied pharmacology, 27(2), 215-224.
6.S. Senda, H. Fujimura, JP 62 4892 (1962), C.A. 59, 642g (1963); E. J. Soboczenski, US 3235360 (1966 to duPont Nemours).
7.Thomas A Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5.