L-Glucose: Uses, Characteristics
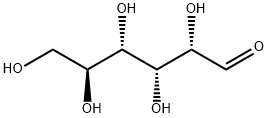
L-glucose is an organic compound and its IUPAC name is (2S,3R,4S,5S)-2,3,4,5,6-pentahydroxyhexanal. Its molecular formula and molecular weights are C6H12O6 and 180.16 g/moL respectively. This compound naturally occurs in fruits and other parts of plants in its free state. However, it is not present in higher living organisms. Compared with D-glucose, the L-glucose is less biologically active and less common. In higher forms of organisms, the L-glucose is not produced naturally. It is synthesized artificially in a laboratory. L-glucose cannot be used as a source of energy in cellular respiration. That is because hexokinase cannot phosphorylate it during glycolysis. Burkholderia caryophylli, though, has the enzyme D-threo-aldose 1-dehydrogenase that enables the oxidation of L-glucose[1].
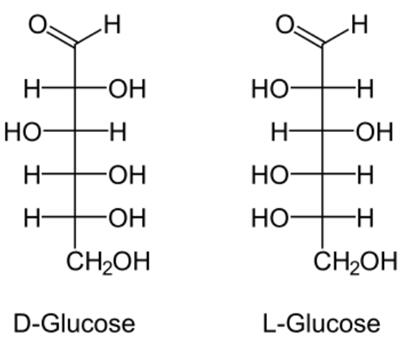
L-Glucose is specifically one of the aldohexose monosaccharides. As the L-isomer of glucose, it is the enantiomer of the more common D-glucose. L-Glucose does not occur naturally in higher living organisms, but can be synthesized in the laboratory. The enzyme system in the human body acts on D-Glucose and is ineffective on L-Glucose. This is because the enzymes are required to catalyze, and the substrate molecules are required to match the enzyme molecules in shape. L-Glucose is not catalyzed. The configuration required for the sugar-metabolizing enzyme is not digested or absorbed or absorbed to a small extent, so there is no energy. L-Glucose is indistinguishable in taste from D-glucose, but cannot be used by living organisms as source of energy because it cannot be phosphorylated by hexokinase, the first enzyme in the glycolysis pathway. One of the known exceptions is in Burkholderia caryophylli, the enzyme D-threo-aldolse dehydrogenase is capable of oxidising L-glucose Like the 'D-' isomer, L-glucose usually occurs as one of four cyclic structural isomers-α- and β-L-glucopyranose, and α- and β-L-glucofuranose. In solution, these isomers interconvert in matters of hours, with the open-chain form as an intermediate stage-a catabolic pathway that can utilize L-glucose has been discovered in Paracoccus laeviglucosivorans[2].
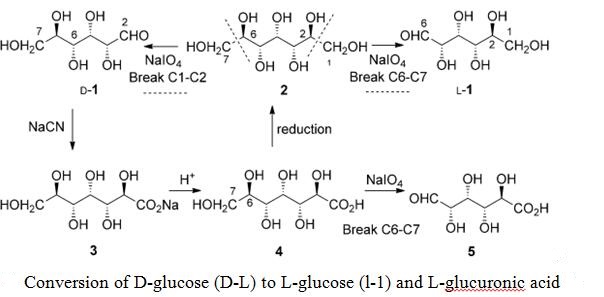
L-glucose is a rare sugar in nature that can be synthesized by chemical conversion of L-arabinose[3]. The isomerization process of L-glucose may be the fixation of cells containing xylose isomerase or disrupted cells by butyl acetate wall separation and sonication. The characteristics of L-glucose are: (1) Does not provide human energy; (2) Has the same taste as D-glucose; (3) Because oral microbes cannot ferment L-glucose, so it does not cause dental caries; (4) Bacterial spoilage and decay are immune; (5) Can be used as a substitute for D-glucose without additional fillers; (6) Stable in aqueous solution; (7) Including foods that require heat treatment stable at work; (8) Can be used in baked goods, can occur Maillard reaction; (9) Suitable for diabetics.
References
[1] Sasajima, K., & Sinskey, A. Oxidation of l-glucose by a Pseudomonad. Biochimica et Biophysica Acta (BBA)-Enzymology, 1979(571): 120-126.
[2] Kazuhiro F , Kunio O , Masaya K , et al. Structural basis of L-glucose oxidation by scyllo-inositol dehydrogenase: Implications for a novel enzyme subfamily classification[J]. PLOS ONE, 2018, 13(5):1-15.
[3] Horwath O,Colonna W J. Process for isomerizing L-glucose to L-fructose: The United States of America,4,463,093[P].1984,July.
You may like
Related articles And Qustion
See also
Lastest Price from L-GLUCOSE manufacturers

US $0.00/KG2025-04-21
- CAS:
- 921-60-8
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1990.00/g2024-06-23
- CAS:
- 921-60-8
- Min. Order:
- 1g
- Purity:
- 97
- Supply Ability:
- 500 Kg