Is Potassium formate an acid or a base?
Aug 15,2024
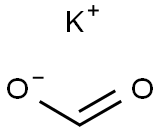
Potassium formate (HCOOK) is basic. HCOOK is a salt formed from a strong base (KOH) and a weak acid (formic acid). Potassium formate is a monocarboxylic anion, a conjugate base of formic acid. Therefore, the pH of the HCOOK solution will be greater than 7 (basic).
The reason for this is as follows:
HCOOK will hydrolyse in water: HCOOK + H2O → HCOOH + KOH
Put another way, HCOO- + H2O → HCOOH + OH- (HCOO- is the conjugate base of HCOOH)
Related articles And Qustion
Lastest Price from Potassium formate manufacturers
Potassium Formate

US $1200.00-1100.00/ton2025-08-12
- CAS:
- 590-29-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M
Potassium Formate

US $1200.00-1100.00/ton2025-08-12
- CAS:
- 590-29-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M