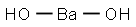Is Ba(OH)2 a strong base?
Strong bases completely dissociate in water into the cation and OH- (hydroxide ion). The hydroxides of the Group I (alkali metals) and Group II (alkaline earth) metals are usually considered strong bases. These are classic Arrhenius bases. Here is a list of the most common strong bases:
Strong Base include LiOH, NaOH, KOH, RbOH, CsOH (Group I)
Ca(OH)2, Sr(OH)2,Ba(OH)2 (Group II).
Strong Acid include HCl, HBr, HI (GroupVII except for HF)
HNO3, H2SO4, HClO4.
As the metal it is made of (barium) sits at the bottom of Group II, its hydroxide is considerably strong and comparable to Group I alkalis.
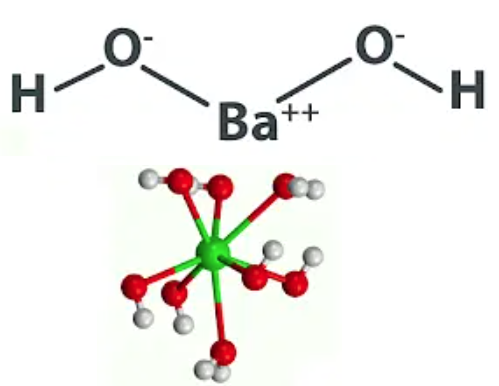
These bases completely dissociate in solutions of 0.01 M or less. The other bases make solutions of 1.0 M and are 100% dissociated at that concentration. There are other strong bases than those listed, but they are not often encountered.
The strong bases are excellent proton (hydrogen ion) acceptors and electron donors. The strong bases can deprotonate weak acids. Aqueous solutions of strong bases are slippery and soapy. However, it's never a good idea to touch a solution to test it because these bases tend to be caustic. Concentrated solutions can produce chemical burns.
In addition to the strong Arrhenius bases, there are also superbases. Superbases are Lewis bases that are Group 1 salts of carbanions, such as hydrides and amides. Lewis bases tend to be stronger than the strong Arrhenius bases because their conjugate acids are so weak. While Arrhenius bases are used as aqueous solutions, the superbases deprotonate water, reacting with it completely. In water, none of the original anions of a superbase remain in solution. The superbases are most often used in organic chemistry as reagents.
You may like
Related articles And Qustion
See also
Lastest Price from Barium hydroxide manufacturers

US $0.00-0.00/kg2025-03-07
- CAS:
- 17194-00-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons

US $0.00-0.00/kg2025-03-07
- CAS:
- 17194-00-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 100tons