Is 1,2-Dimethoxyethane a polar or non-polar compound?
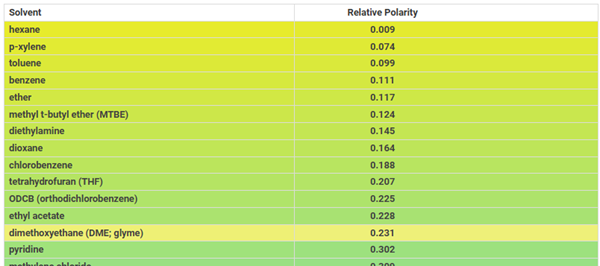
1,2-Dimethoxyethane (DME) is a non-polar compound with a relative polarity of 0.231 (see figure below) and is slightly soluble in water.
The conformation of 1,2-dimethoxyethane in the liquid phase and in aqueous solutions was studied by Raman spectroscopy. The conformation-sensitive Raman bands of 1,2-dimethoxyethane, located in the wavenumber region 300?600 cm-1, were analyzed by means of the band decomposition based on normal coordinate analysis. The populations of the conformers of 1,2-dimethoxyethane, their relative energies, and their relative entropies in the liquid phase were estimated and compared with the relevant data for the gas phase of 1,2-dimethoxyethane. The condensed phase effects on the conformation of 1,2-dimethoxyethane were discussed on the basis of the experimental data. The populations of the conformers at 318 K in aqueous solutions with different concentrations were estimated. It was revealed that the populations of the TTT and TGG' conformers of 1,2-dimethoxyethane decrease on increasing concentration of water, while the populations of the TGT and TGG conformers increase. The entropies of the less hydrophilic TTT and TGG' conformers are higher than the entropies of the more hydrophilic TGT and TGG conformers. This experimental observation suggests that the inverse temperature solubility of poly(oxyethylene) in water originates from the conformational changes of the polymer chain.
1,2-Dimethoxyethane (DME) is a good multifunctional solvent with low dielectric constant and high DN (Donor Number) properties and can be used as a solvent for conductivity studies of lithium ion solutions. In addition, the synthesis efficiency of tetrachloro thorium tetrachloride bis(dimethoxyethane), ThCl4(DME)2 can be improved by adding DME to the reaction mixture. This finding is evidenced by the known syntheses of the metallocene dichlorides (C5Me5)2ThCl2 (1), (1,2,4-tBu3-C5H2)2ThCl2 (2) and (C5Me4Et)2ThCl2 (3).
References:
[1] NIKOLAY GOUTEV Hiroatsu M Keiichi Ohno. Raman Spectroscopic Study on the Conformation of 1,2-Dimethoxyethane in the Liquid Phase and in Aqueous Solutions[J]. The Journal of Physical Chemistry A, 2000, 104 40: 9057-9280. DOI:10.1021/jp001340+.[2] M. YOSHIO. Conductivities of 1,2-dimethoxyethane or 1,2-dimethoxyethane-related solutions of lithium salts[J]. Journal of Power Sources, 1993, 41 1: 1-221. DOI:10.1016/0378-7753(93)85006-A.
[3] MARISA J. MONREAL. Enhancing the synthetic efficacy of thorium tetrachloride bis(1,2-dimethoxyethane) with added 1,2-dimethoxyethane: Preparation of metallocene thorium dichlorides[J]. Inorganic Chemistry Communications, 2014, 46: 1-344. DOI:10.1016/j.inoche.2014.04.028.
Related articles And Qustion
Lastest Price from 1,2-Dimethoxyethane manufacturers

US $10.00/KG2025-05-28
- CAS:
- 110-71-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50 ton

US $1.00/KG2025-04-21
- CAS:
- 110-71-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt