Important applications of calcium phosphate
Introduction
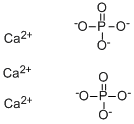
Calcium phosphate, chemical formula Ca3(PO4)2, is a white crystal or amorphous powder[1], insoluble in ethanol and acetone, insoluble in water, and easily soluble in dilute hydrochloric acid and nitric acid. Calcium phosphate is generally slightly harmful to water bodies. Do not contact undiluted or large quantities of product with groundwater, water courses or sewage systems. Do not discharge calcium phosphate into the surrounding environment without government permission. Calcium phosphate is commonly used as an anti-caking agent, acidity regulator, nutritional supplement, flavor enhancer, stabilizer, and moisture keeper.

Picture 1 The physical map of calcium phosphate
Preparation of calcium phosphate
Calcium phosphate exists in apatite and ashes[2]. It can be obtained from calcium chloride and sodium phosphate (in the presence of excess ammonia) or from slaked lime and phosphoric acid. In the cyclone furnace, the phosphate rock is added with an appropriate amount of additives, and the defluorination reaction is carried out with the action of water vapor under the high temperature melting condition. The molten material is quenched and quenched by water, and then dried and ground to obtain the product. Or mix phosphate rock powder with a small amount of sodium carbonate (or sodium sulfate) and a small amount of wet-process phosphoric acid, calcinate in a rotary kiln or a boiling furnace at 1350 °C (use oil or natural gas as the dye), and grind the frit after cooling. It is quenched to α-calcium phosphate at above 1180°C, and slowly cooled to β-form calcium phosphate below 1180°C.
Application of calcium phosphate in biology
Calcium phosphate precipitation is a transfection method for introducing DNA into eukaryotic cells based on calcium phosphate-DNA complexes, which are believed to facilitate the binding of exogenous DNA to the surface of target cells. The calcium phosphate-DNA complex adheres to the cell membrane and enters the target cell via pinocytosis, and the transfected DNA can be integrated into the target cell's chromosome to generate stable clones with different genotypes and phenotypes. This method was first used by Graham and vander Ebb and later modified by Wigler. Can be widely used to transfect many different cell types, not only suitable for transient expression, but also to generate stable transformation products.
Applications of calcium phosphate in food
In the food industry, it is used as an anticaking agent, acidity regulator, nutritional supplement, flavor enhancer, stabilizer, and moisture retention agent. Calcium phosphate can be used for wheat flour, the maximum dosage is 0.03g/kg; solid beverages, 8.0g/kg; fried potato chips, 2.0g/kg. Can be used in flour as a humectant. FAO/WHO regulations: As an anticaking agent, it can be used for glucose powder and sucrose powder, with a maximum dosage of 15g/kg (alone or in combination with other anti-caking agents, without starch); milk powder, cream powder, 5g/kg (alone or in combination with other anti-caking agents). Or combined with other stabilizers, based on anhydrous); when used in vending machines, milk powder, 10g/kg, cream powder 1g/kg (alone or in combination with other anti-caking agents); soup and soup, 15mg/kg (alone or in combination with stearate and silicon dioxide, referring to dehydrated products); cocoa powder and sugar-sweetened cocoa powder, 10g/kg (alone or in combination with other anti-caking agents, sugar-sweetened cocoa powder for vending machines only ). As a stabilizer, it can be used for evaporated milk, sweetened condensed milk, and whipped cream. 9 g/kg (total phosphate, calculated as phosphorus) for processing cheese. This product can still be used as a calcium element fortifier for biscuits, bread, etc. Its dosage is 3g/kg for cereal flour and 20g/kg for solid beverages, all calculated as elemental calcium.
Handling and storage considerations
Calcium phosphate needs airtight operation, pay attention to ventilation during operation. Operators must undergo special training and strictly abide by operating procedures. It is recommended that operators wear self-priming filter dust masks. Avoid calcium phosphate dust. Avoid contact of calcium phosphate with acids. When handling calcium phosphate, it should be lightly loaded and unloaded to prevent package damage. Equipped with leakage emergency treatment equipment. Empty calcium phosphate containers may contain harmful residues. Calcium phosphate should be stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Calcium phosphate should be stored separately from acids, and should not be mixed. Calcium phosphate storage areas should be provided with suitable materials to contain spills.
Reference
1 Zheng Junmin. Handbook of Pharmaceutical Excipients. Beijing: Chemical Industry Press, 2004.7
2 Li Xiang. Food Additive Use Technology: Chemical Industry Press, 2010: 299
Related articles And Qustion
See also
Lastest Price from Calcium phosphate manufacturers

US $1200.00-1100.00/ton2025-09-18
- CAS:
- 7758-87-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $0.00/KG2025-04-21
- CAS:
- 7758-87-4
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month