How to synthesize Brexanolone?
Introduction
Postpartum depression (PPD) affects 10−20% of women worldwide and is one of the primary contributors to maternal morbidity. Additionally, PPD poses severe risks to the emotional, cognitive, behavioral, and physical development of the infant and siblings. Brexanolone is a β-cyclodextrin based allopregnanolone formulation that helps maintain stable physiological serum concentration. Developed by Sage Therapeutics, brexanolone was approved by the USFDA as a first-in-class treatment for PPD in adult women[1].
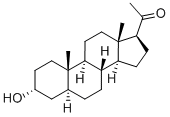
Brexanolone is a formulation of the naturally occurring neuroactive steroid allopregnanolone, which, despite extensive study, had not been approved for use as a therapeutic prior to 2019. Allopregnanolone has low aqueous solubility, poor oral bioavailability, and low metabolic stability. The drug is a positive allosteric modulator (PAM) of synaptic and extrasynaptic γ-aminobutyric acid type A (GABAA) receptors. The plasma concentration of allopregnanolone increases in concert with progesterone during pregnancy and reaches its highest level in the third trimester. However, its concentration falls abruptly after childbirth. Failure of GABAA receptors to adapt to these sudden changes triggers postpartum depression.
Synthesis method
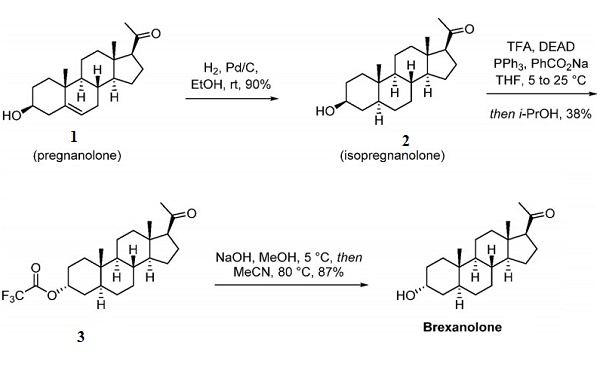
Brexanolone was prepared on a scale via the semisynthetic pathway depicted above. The synthesis began from pregnenolone (1), another naturally occurring steroid—catalytic hydrogenation of compound 1 produced isopregnanolone (2), an epimer of allopregnanolone. Stereoinversion at the 3β-hydroxy center was accomplished via the Mitsunobu reaction. Alcohol 2 was treated with trifluoroacetic acid in the presence of diethyl azodicarboxylate (DEAD) and triphenylphosphine to generate stereoinverted trifluoroacetate 3. In this case, the Mitsunobu reaction byproduct, triphenylphosphine oxide, was precipitated from diisopropyl ether before the filtrate concentration. Recrystallization of the product furnished trifluoroacetate 3 in high purity without column chromatography. Finally, saponification furnished brexanolone, which was purified by recrystallization from hot acetonitrile.
References
[1] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
See also
Lastest Price from Allopregnanolone manufacturers
US $0.00-0.00/kg2025-08-22
- CAS:
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1

US $24.00-2.00/g2025-06-18
- CAS:
- 516-54-1
- Min. Order:
- 1g
- Purity:
- 99.958%
- Supply Ability:
- 200000G