How to synthesis Cesium iodide colloidal NCs?
Introduction
Cesium iodide (CsI) is a kind of ionic compound that has been widely used as scintillators in many areas such as X-ray image intensifier tubes, photocathodes, and display devices. Current studies of CsI scintillators have been mostly focused on monocrystalline CsI columns or polycrystalline CsI films deposited on substrates. The size reduction of CsI from bulk crystals to NCs possibly leads to three-dimensional confinement and more overlap of electron and hole wavefunctions, resulting in optical transitions with higher efficiency and faster rates. Therefore, the use of CsI NCs as nanoscitillators is expected to eliminate the issue of slow response of conventional scintillator detectors, and the excellent solubility of CsI colloidal NCs in organic solvents also enables solution-processable devices with low cost.
However, the controlled synthesis of high-quality CsI colloidal NCs with tunable morphologies still remains a challenging task, due to its ionic characteristics as mentioned above. On the other hand, CsI is a kind of electron sensitive material, exhibiting obvious degradation under electron beam irradiation. For example, the grain boundaries (known as “dead areas”) of CsI films were reported to present white spots under continuous electron beam irradiation. Such small CsI NCs possess large specific surface area, and thus it is of great significance to explore the electron beam-induced transformations of CsI NCs.
Synthesis
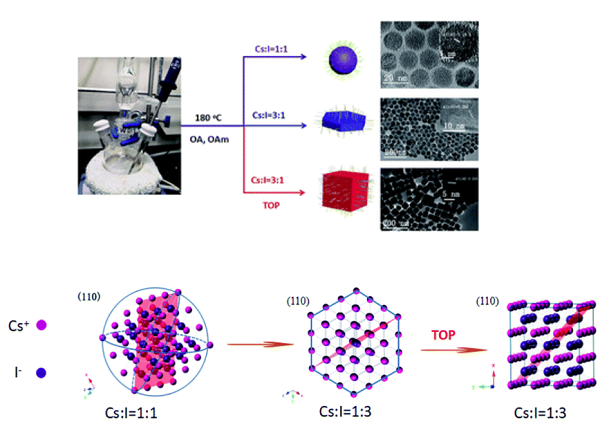
The solution synthesis of CsI colloidal NCs is based on the hot-injection method that is often adopted to prepare a series of nanomaterials, and the reactions are performed under air-free conditions using standard Schlenk technique. CsI NCs are prepared by reacting cesium oleate (Cs-OA) with germanium iodide (GeI2) in a high boiling solvent octadecene (ODE), in the presence of certain coordinating ligands. By manipulating the ratio of I− and Cs+ precursors (from 1 to 3) and the ligand categories, CsI nanospheres, hexagonal nanoplates and nanocubes were synthesized. As the co-precipitation reaction of Cs+ and I− proceeding, the solution color changes from colorless to fawn and then to white, indicating the formation of CsI NCs. The optimal reaction temperature is 180 °C, as there are no products below this temperature and CsI NCs with irregular shapes appear at higher temperature.
Synthesis of spherical CsI NCs
GeI2 precursor solution was prepared by dissolving 0.01 g GeI2 with 2 mL ODE, 0.2 mL OA and 0.2 mL OAm in a vial protected by an Argon gas, which is heated to 100 °C in an oil bath. ODE (3 mL), OA (0.1 mL) and Cs2CO3 (0.015 g) were loaded into a 100 mL 3-neck flask and vacuumed at 110 °C for 1 h unceasingly. After complete solubilization of Cs2CO3, the temperature was raised to 180 °C, and then as-prepared GeI2 solution was swiftly injected, and the reaction mixture was cooled down 5 min later by a water bath.
Synthesis of hexagonal CsI NCs
The same procedure as that for spherical CsI NCs was followed, except that the quantity of the GeI2 was increased to 0.03 g, and the reaction time was increased to 10 minutes.
Synthesis of cubic CsI NCs
The same procedure as that for hexagonal CsI nanoplates was followed, except that the addition 1 mL TOP was introduced into the dissolution process of GeI2.
References
[1] Song, Weidong et al. “Morphologically controlled synthesis of ionic cesium iodide colloidal nanocrystals and electron beam-induced transformations†.” RSC Advances 33 (2018): 18519–18524.
You may like
See also
Lastest Price from Cesium iodide manufacturers

US $10.00/ASSAYS2025-08-29
- CAS:
- 7789-17-5
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 1 ton

US $0.00/KG2025-04-21
- CAS:
- 7789-17-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month