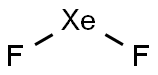How the XeF4 lewis structure is formed
XeF4 lewis structure
The XeF4 Lewis structure consists of a central atom, xenon (Xe), and four outer atoms, fluorine (F), bonded at 90° and 180°. The xenon atom (Xe) and each fluorine atom (F) are connected by a single bond. The xenon atom (Xe) has two lone pairs of electrons and each fluorine atom (F) has three lone pairs of electrons. The Lewis structure of XeF4 is shown below:
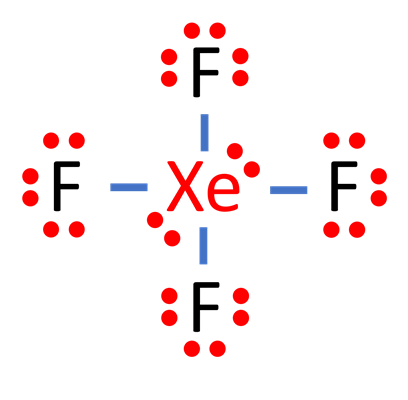
Steps for drawing the XeF4 Lewis structure
Step 1 Calculate the number of valence electrons for Xe and F
Xenon and fluorine are elements of group 18 and 17 of the periodic table, respectively. Therefore, xenon and fluorine atoms have 8 and 7 valence electrons, respectively, so the total number of valence electrons in the XeF4 molecule = 1 valence electron from xenon + 4 valence electrons from fluorine = 8 + 7(4) = 36.
Step 2 Identify the central atom
The central atom must be highly or minimally electronegative. For the XeF4 molecule, fluorine (F) is the most electronegative atom in the periodic table, whereas xenon (Xe) is less electronegative than fluorine, so xenon is the central atom and fluorine is the outer atom.
Step 3 Labelling the electron lone pairs between atoms
Total number of valence electron pairs = σ-bonds + π-bonds + lone pairs of electrons in the valence layer, i.e., the total number of valence electron pairs divided by 2. In the case of the XeF4 molecule, the total number of electron pairs is 18.
For the XeF4 molecule, the total number of pairs of electrons is 18. The xenon atom is connected to each fluorine atom by a σ-bond (one σ-bond equals one pair of electrons) to form a total of four σ-bonds, and the remaining 14 pairs of electrons are distributed as follows: two lone pairs of electrons on the xenon atom, and three lone pairs of electrons on each of the fluorine atoms (3).
Step 4 Stability of structure
In order to make the Lewis structure of the XeF4 molecule more stable, we have to check if an octet is formed in the XeF4 molecule. In step 3, there are three lone pairs of electrons on each of the external fluorine atoms (F), and the fluorine atoms are connected to the central xenon atom (Xe) by a σ-bond, which is also a pair of lone pairs of electrons, so that there are eight electrons around the fluorine atoms (F), forming an octet of stable structure. xenon (Xe) does not follow the octet rule. It has a total of 12 valence electrons in the Lewis structure of XeF. During chemical bonding, incident valence electrons can enter the 4d subshell layer of Xe, thus facilitating the accommodation of more than eight valence electrons.