How the N3-lewis structure is formed?
N3-Lewis Structure
The Lewis structure of N3- (azide ion) is composed of three nitrogen atoms (N). There are 2 double bonds between each nitrogen atom (N). There are two lone electrons on each of the two outer nitrogen atoms. The overall external appearance of the molecule shows a -1 form of charge. The N3- Lewis structure is shown below:
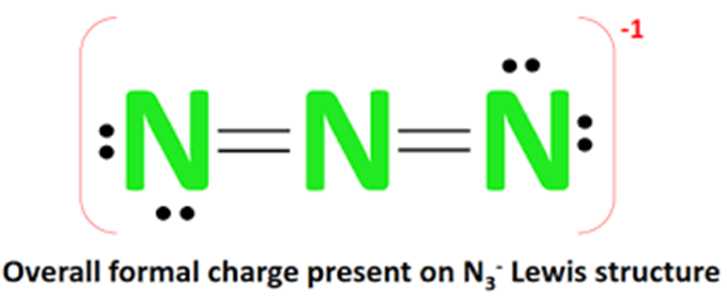
Steps for drawing the N3- Lewis structure
Step 1 Calculate the number of valence electrons for N
Nitrogen is in group 15 of the periodic table. As a result of this, nitrogen has five valence electrons. Because N3– contains three nitrogen atoms, Three nitrogen atoms'valence electrons = 5 × 3 = 15 Because the N3– now has a negative (-1) charge, we must add another electron. As a result, there are 15 + 1 = 16 valence electrons altogether.
Step 2 Identify the central atom
The central atom must be highly or minimally electronegative. For the N3-molecule, all three atoms are identical, so you can choose any atom as the central atom.
Step 3 Labelling the electron lone pairs between atoms
Total number of valence electron pairs = σ-bonds + π-bonds + lone pairs of electrons in the valence layer, i.e., the total number of valence electron pairs divided by 2. In the case of the N3- molecule, the total number of pairs of electrons is eight. A central nitrogen atom is connected to two other nitrogen atoms by a σ-bond (one σ-bond equals one pair of electrons). Because nitrogen is a period 2 element, it can only have 8 electrons in its final shell. Always begin by identifying lone pairs from exterior atoms. The outer atoms are nitrogen, both left and right. So there are three lone pairs for left and right nitrogen, and zero lone pairs for canter nitrogen because all six electron pairs have been used up.
Step 4 Stability of structure and minimize charges on atoms by converting lone pairs to bonds
When there are positive and negative charges on lot of atoms or higher charges (like +2, +3, -2, -3) on atoms in an ion or molecule, that structure is not stable. Therefore, We should try to reduce charges on atoms if it is a possible.
In step 3 we can see that the external atomic nitrogen atoms form an octet, so they are stable. However, we must check that the central nitrogen atom (N) is stable and we can see that the central nitrogen atom is unstable with only 4 electrons, now to make this nitrogen atom stable the electron pair must be moved away from the outer nitrogen atom so that the central nitrogen atom can have 8 electrons (i.e. an octet). By removing one electron pair from each of the outer nitrogen atoms, the centre nitrogen atom can form an octet.
Upon movement, the two nitrogen atoms each form two double bonds with the central nitrogen atom, retaining two lone electrons on each of the outer nitrogen atoms, and since N3- has a negative (-1) charge, brackets are added to the Lewis structure to indicate that charge.
N3– Hybridization
To determine the hybridization of the central atom in the Azide ion, it is pertinent that we observe its Lewis structure discussed above. The central Nitrogen atom is chemically bonded to two adjacent Nitrogen atoms through double bonds. As we've discussed the concept of electron regions earlier, we can quickly determine the hybridization from this data. Two regions are surrounding the central Nitrogen atom. Therefore, the hybridization of the Azide ion is determined to sp.
N3– Molecular Geometry
The Azide Lewis structure comprises three Nitrogen atoms. The central Nitrogen atom forms two double bonds with the adjacent nitrogen atoms. According to the VSEPR theory, the atoms will repel each other to give a Linear Geometry.