How do you make citric acid?
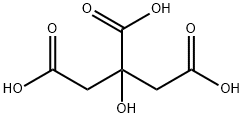
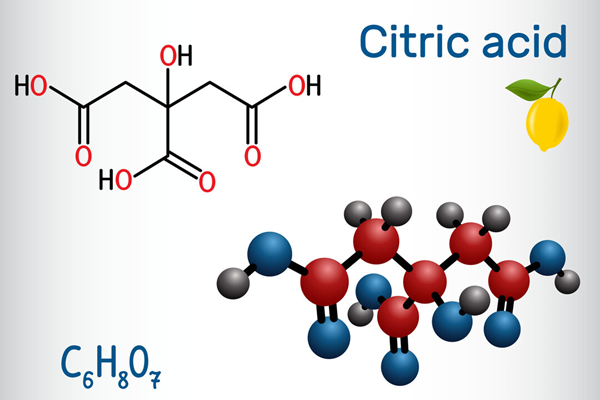
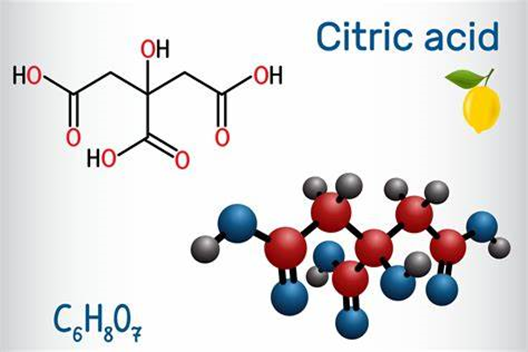
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. Usually encountered as a white solid, it is a weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.

How do you make citric acid?
Citric acid is found naturally in citrus fruits, but producing citric acid from citrus fruits is very expensive and the demand for citric acid is greater than the available supply of citrus fruits. Therefore, when you see citric acid on a product label, you can be sure that it is a powder that was made from the fermentation of sugars.
A culture of Aspergillus niger (a fungus commonly used in the pharmaceutical industry) is fed with sugar and metabolizes it into a liquid solution. The solution is mixed with lime (calcium hydroxide) which causes citrate salt to come out of the solution (precipitate). The citrate salt is then treated with sulfuric acid to make useable citric acid.
The sugars that are used for the citric acid can be derived from cane sugar, corn or wheat. In the United States, citric acid is most often derived from corn since it is a cheap, subsidized crop. In South America cane sugar is often used due to the low sugar prices, while in Europe wheat sweeteners are commonly used.
Application
It is a natural preservative and is also used to add an acidic (sour) taste to foods and soft drinks.
In biochemistry, it is important as an intermediate in the citric acid cycle and therefore occurs in the metabolism of almost all living things.
It also serves as an environmentally benign cleaning agent and acts as an antioxidant.
Citric acid exists in a variety of fruits and vegetables, but it is most concentrated in lemons and limes, where it can comprise as much as 8 percent of the dry weight of the fruit.
Safety
Although a weak acid, exposure to pure citric acid can cause adverse effects. Inhalation may cause cough, shortness of breath, or sore throat. Over-ingestion may cause abdominal pain and sore throat. Exposure of concentrated solutions to skin and eyes can cause redness and pain. Long-term or repeated consumption may cause erosion of tooth enamel.
Related articles And Qustion
See also
Lastest Price from Citric acid manufacturers

US $0.00-0.00/kg2025-12-09
- CAS:
- 77-92-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $1.00-0.50/kg2025-11-03
- CAS:
- 77-92-9
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 200tons