General Introduction of Calcium Carbonate Powder
Calcium carbonate is a common substance found in rocks as the minerals calcite and aragonite (most notably as limestone, which is a type of sedimentary rock consisting mainly of calcite) and is the main component of eggshells, snail shells, seashells and pearls. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues.

Physical Properties
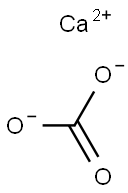
Calcium carbonate is an inorganic compound, the main component is limestone, the molecular formula is CaCO3, and the molecular weight is 100.09. Calcium oxide accounted for the highest proportion, 56.03%, followed by carbon dioxide 43.97%. Due to the characteristics of calcium carbonate, it exists widely in nature and is used in various industries with its unique advantages.
Calcium Carbonate Types
Calcium carbonate is classified from the perspective of production methods, including activated calcium carbonate, light calcium carbonate and heavy calcium carbonate. It occupies active calcium carbonate, which is surface calcium carbonate treatment, colloidal calcium carbonate, which is obtained by treating heavy calcium carbonate or light calcium carbonate with surface modifier. Strong effect, that is, active effect. Light calcium carbonate, commonly known as light calcium, is calcined as raw material of limestone to form lime and carbon dioxide, which is added to react with water to form lime milk, connected with carbon dioxide to form calcium carbonate precipitation, and formed after a series of processes such as dehydration, drying and pulverization. Heavy calcium carbonate is physical grinding and crushing of marble, limestone, dolomite and calcite through Raymond mill, vertical mill, ball mill and other equipment to obtain 100-3000 mesh powder, and the sedimentation volume is smaller than that of light calcium carbonate.
Application
Calcium carbonate powder is an important inorganic chemical product, widely used in rubber, plastic, paint, paper making, food, cosmetics, medicine and daily chemical industries. Because the surface of calcium carbonate has many hydroxy groups, the surface is hydrophilic and hydrophobic, easy to form aggregates, poor dispersion performance, directly affect the application. Such as carbon Chemicalbook calcium acid used in polymer or rubber products, it can not be chemically combined with the polymer, it is difficult to play a reinforcing role, but can only play a capacitive role. Therefore, due to the filling of calcium carbonate will cause some of the polymer performance decline, especially excessive filling, will make the polymer performance sharply decreased, so that the product can not be used.
Production Method
Reaction of calcium hydroxide and hydrochloric acid to produce calcium chloride, decolorization by activated carbon, filtration to remove impurities, so that calcium chloride in the presence of ammonia carbonization with carbon dioxide to obtain calcium carbonate, and then through crystallization, separation, washing, dehydration, drying, screening, crystal calcium carbonate products:
CaCO3→CO2+CaO
caO+H2O→Ca(OH)2
Ca(OH)2+CO2→CaCO3+H2O
You may like
Related articles And Qustion
Lastest Price from Calcium carbonate manufacturers
US $0.00-0.00/kg2025-12-13
- CAS:
- 471-34-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000

US $1200.00-1100.00/ton2025-09-10
- CAS:
- 471-34-1
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M