General Description of Imidazole
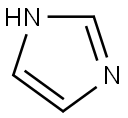
Imidazole is a planar, five-membered, unsaturated, 6π electron ring system, comprised of three carbon atoms and two nitrogen atoms at 1,3-positions with two olefinic bonds. One of the nitrogen atoms, N1 , attached to hydrogen is a pyrrole type, while N3 is a pyridine type. The delocalized lone pair of electrons of N1 nitrogen participates in the π sextet and is less basic than pyridine nitrogen, which contributes only one electron to the aromaticity leaving a lone pair in the sp2 hybrid orbital, responsible for its basic characteristic. The presence of N3 (pyridine type) in the ring lowers the energy level of π orbitals as evident from its ionization potential, which is higher than pyrrole, thiophene, and furan. At the same time, the negative inductive effect of nitrogen provides stabilization to a negatively charged reaction intermediate (nucleophile). The π electrons of the ring system are delocalized although electron density is concentrated on both the nitrogen atoms and characterized as π-excessive heterocycles. The presence of a lone pair of electrons on N3 is responsible for easy protonation in strong acid, an indication of a strong base. The formation of imidazolide in a strong base is also an indication of strong acid. Thus imidazole is amphoteric in nature.
Physical Properties
It is a hygroscopic white crystalline solid with an ammonia-like odor, an mp of 88–91°C, and a bp of 256°C. It has amphoteric properties, a density of 1.0303 gm/mL, a viscosity of 2.696, and a flash point of 145°C. It is soluble in benzene, ether, acetone, petroleum ether, chloroform, pyridine, and water.
UV (ethanol) λnm (ε): 207 (3.70).
1H NMR (CDCl3 ), δ (ppm): C2 –H, 7.73; C4 –H, 7.14; C5 –H, 7.14.
13C NMR (CDCl3 ), δ (ppm): C2 , 135.4; C4 , 121.9; C5 , 121.9.
The calculated ionization energy of imidazole is 8.78 eV, while its dipole moment is 3.70 D in the gas phase. The resonance energy of imidazole is 14.2 kcal/mol. The bond length is in angstroms, the bond angle is in degrees, and electron density distribution around the ring is depicted in the following diagram.
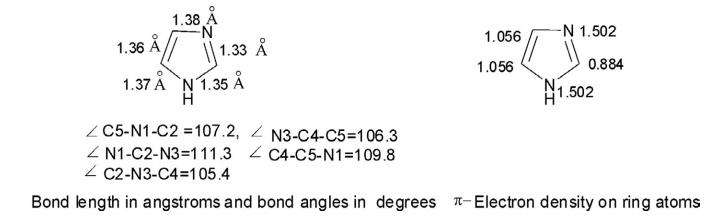
As evident from the calculated bond angle, N1C2N3 (111.3 degrees) is largest, while the angle at C2-N3-C4 (105.4 degrees) is smallest. The bond length between C4N3 (1.38 Å) is largest, while C2-N3 (1.33 Å) is smallest. The C4-C5 (1.36 Å) bond length is a little longer than the normal C-C bond length.
The electron density map reveals that electron density at N1 and N3 is 1.503, while at C4 and C5 it is 1.056. Thus the C4 - and C5 -positions are equally vulnerable to electrophiles, while site C2 (0.884) is prone to nucleophilic attack because its electron density is lowest.
Imidazole without substitution at nitrogen also exists in two possible tautomeric forms A and B and is in rapid equilibrium. Thus the task of separation of the tautomers becomes very difficult.

As evident from the calculated bond angle, N1-C2-N3 (111.3 degrees) is largest, while the angle at C2-N3-C4 (105.4 degrees) is smallest. The bond length between C4-N3 (1.38 Å) is largest, while C2-N3 (1.33 Å) is smallest. The C4-C5 (1.36 Å) bond length is a little longer than the normal C-C bond length. The electron density map reveals that electron density at N1 and N3 is 1.503, while at C4 and C5 it is 1.056. Thus the C4 - and C5 -positions are equally vulnerable to electrophiles, while site C2 (0.884) is prone to nucleophilic attack because its electron density is lowest.
Imidazole without substitution at nitrogen also exists in two possible tautomeric forms A and B and is in rapid equilibrium. Thus the task of separation of the tautomers becomes very difficult.

The tautomeric equilibrium under specific conditions can be shifted mainly toward one of the two forms. Introduction of electron-withdrawing substituents predominantly favors the 4-substituted tautomer. In neutral organic solvents, equilibrium is attained by an intermolecular process, involving two or more imidazole moieties through hydrogen bonding. Imidazole is a good donor and acceptor of hydrogen bonds. The pyridine-like nitrogen (N3 ) is an electron donor, while the pyrrole type of nitrogen (N1 ) is an acceptor.

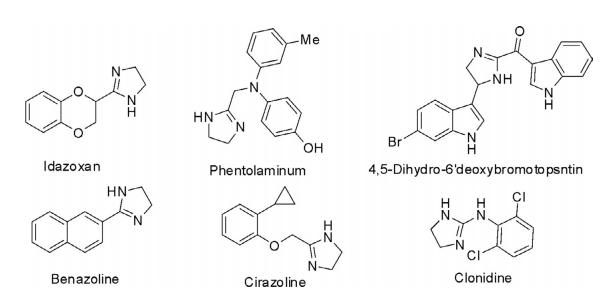
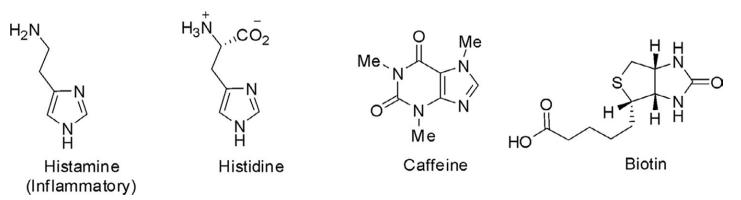
The imidazole ring is present in various natural products in isolated form such as histamine and histadine, a natural amino acid. It is also present as a substructure in fused ring systems such as caffeine and biotin.
Natural Products of the Imidazol Ring System
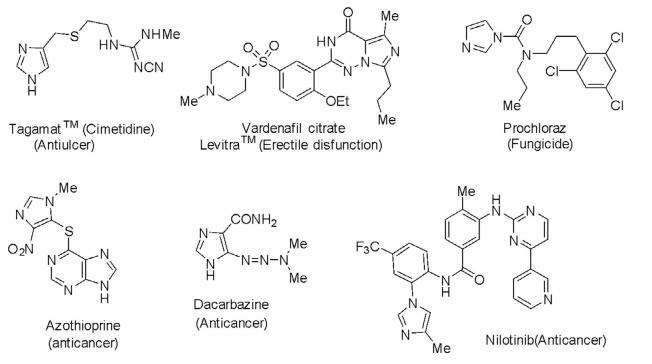
Imidazoles either in isolated form or as a substructure in fused ring systems are of high therapeutic importance and numerous drugs are in clinical use for the treatment of various ailments. Some of the important clinically used drugs are mentioned in the following diagram.
Synthesis
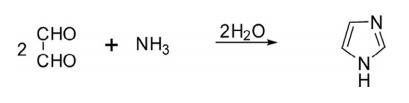
(a) The synthesis of imidazole was first reported in 1858 from the condensation of glyoxal and ammonia in low yields due to side product formation.
(b) An imidazole derivative has also been obtained by the reaction of glyoxal, formaldehyde, and hydroxylamine in methanol at 0°C.

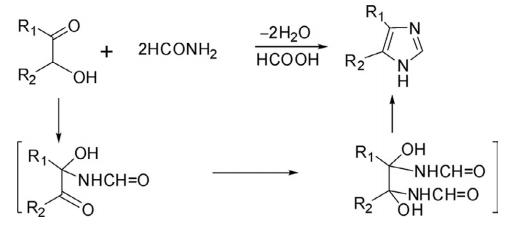
A most general method for the synthesis of parent, mono-, di-, and trisubstituted imidazoles has been reported by Radziszewski through the condensation of 1,2-dicarbonyl compounds such as glyoxal, α-ketoaldehyde, and α-ketoketone with aldehyde in the presence of ammonia-H2 O or NH4 Ac-AcOH.

Imidazole derivatives have also been prepared through modified Radziszewski reaction by heating α- hydroxyketone or an α-haloketone with formamide. This method provides a route for the construction of 4- and 4,5-disubstituted imidazoles.
Another classical route reported by Wallach for the construction of imidazoles is through cyclization of N,N′- disubstituted oxamides with PCl5 to deliver 1-substituted 5-chloroimidazoles.
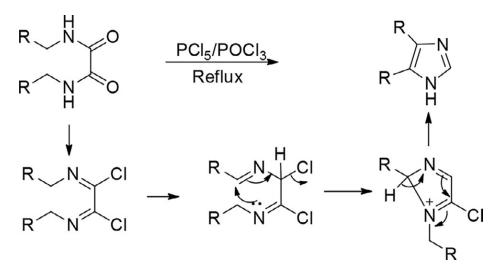
Chemical Reactivity
Imidazole is a π-excessive heterocycle and undergoes electrophilic substitution reactions smoothly. With some manipulation in substitution pattern in the imidazole ring, nucleophilic substitution is also facilitated. The presence of a pyridine-like nitrogen (N3 ) has a lone pair of electrons, responsible for electrophilic substitution at N3 . The other nitrogen (N1 ) is a pyrrole type and its lone pair of electrons involved in the aromatic sextet facilitates electrophilic substitution at carbon atoms C4 and C5 because of higher electron density compared to C2.
You may like
Related articles And Qustion
See also
Lastest Price from Imidazole manufacturers

US $5.00/kg2025-06-10
- CAS:
- 288-32-4
- Min. Order:
- 10kg
- Purity:
- 99.7%
- Supply Ability:
- 10000kg

US $1.00/KG2025-04-21
- CAS:
- 288-32-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt