Gatifloxacin: Pharmacodynamic Properties, Dosage and Administration
General Description
Gatifloxacin, an 8-methoxy fluoroquinolone, exhibits potent antibacterial activity through its dual mechanism of inhibiting bacterial DNA gyrase and topoisomerase IV. This broadens its efficacy against various Gram-positive and Gram-negative organisms, including atypical pathogens. Clinical studies demonstrate Gatifloxacin’s effectiveness in treating infections like otitis media and pneumonia, with minimal resistance development due to the need for multiple mutations for high resistance. It is available in oral and intravenous forms, with a recommended dosage of 400 mg for most indications. Special considerations include dosage adjustments for renal impairment and caution in specific patient populations to ensure safety and efficacy.

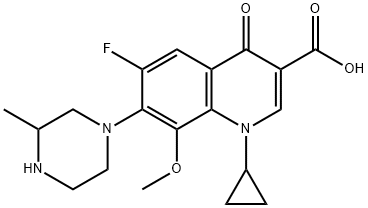
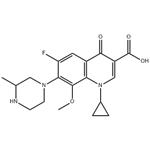
Figure 1. Gatifloxacin
Pharmacodynamic Properties
Overview
Gatifloxacin, an 8-methoxy fluoroquinolone, demonstrates remarkable pharmacodynamic properties that contribute to its antibacterial activity. The drug operates through a dual mechanism by inhibiting both bacterial DNA gyrase and topoisomerase IV, which are essential enzymes for bacterial DNA replication and transcription. This dual site of action allows Gatifloxacin to exhibit a broader spectrum of antibacterial activity compared to earlier fluoroquinolones like ciprofloxacin. Its efficacy covers many Gram-positive and Gram-negative organisms, making Gatifloxacin a potent antibiotic choice in treating various bacterial infections. Atypical organisms, mycobacteria, and some anaerobic bacteria also exhibit susceptibility to Gatifloxacin, further enhancing its clinical utility. 1
Efficacy Against Gram-Positive and Atypical Organisms
In multiple in vitro studies, Gatifloxacin showed superior activity against Gram-positive organisms in comparison to ciprofloxacin. For instance, SENTRY data indicates Gatifloxacin's minimum inhibitory concentration (MIC90) values for strains such as Staphylococcus epidermidis and Streptococcus pneumoniae are ≤2 mg/L and 0.5 mg/L, respectively. In a noteworthy Canadian study involving over 4,000 clinical isolates, Gatifloxacin effectively inhibited a wide range of Gram-positive bacteria, exhibiting MIC90 values that generally ranged from 0.25 to 16 mg/L, although it displayed reduced activity against Enterococcus faecalis and vancomycin-resistant enterococci. Additionally, Gatifloxacin has shown effectiveness against atypical organisms such as Mycoplasma hominis and Chlamydia pneumoniae, demonstrating MIC90 values as low as 0.25 mg/L, establishing it as a robust option for treating infections caused by these pathogens. 1
Resistance Mechanisms and Clinical Implications
The development of resistance to fluoroquinolones, including Gatifloxacin, is primarily mediated through mutations in target sites (DNA gyrase and topoisomerase IV) or mechanisms that decrease drug accumulation within the bacteria. Notably, the need for multiple mutations across different genes for highly resistant strains indicates that Gatifloxacin maintains its effectiveness even against some resistant organisms. The post-antibiotic effect (PAE) of Gatifloxacin ranges from 0.5 to 4.1 hours across various bacteria, suggesting sustained activity post-treatment. Furthermore, pharmacokinetic studies indicate that clinically achievable concentrations of Gatifloxacin exceed the MICs for relevant pathogens over a 24-hour period, enhancing its therapeutic profile. Clinical studies have validated Gatifloxacin’s efficacy in treating a variety of infections, including otitis media and pneumonia, positioning it as a critical agent in the antibiotic arsenal against both common and challenging infections. 1
Dosage and Administration
Administration Guidelines
Gatifloxacin is available in both oral and intravenous formulations in various countries, although only the oral form is currently available in Europe. When administering oral Gatifloxacin, it can be taken without regard to meals, ensuring adaptability for patients. Importantly, there is no need for dosage adjustment when switching between intravenous and oral formulations of Gatifloxacin, which simplifies the treatment regimen for healthcare providers. For uncomplicated urinary tract infections (UTIs), Gatifloxacin is typically prescribed at a single dose of 400 mg, or alternatively, 200 mg taken once daily for three days. In all other indications, the recommended dosage is 400 mg once daily, with varying treatment durations depending on the specific condition. 2
Special Considerations
In patients with moderate to severe renal dysfunction (creatinine clearance <40 ml/min) or those undergoing hemodialysis or continuous ambulatory peritoneal dialysis (CAPD), the dosage of Gatifloxacin should be adjusted following established guidelines. For patients receiving hemodialysis, Gatifloxacin should be administered after the dialysis procedure to ensure optimal drug levels. Notably, Gatifloxacin is not recommended for use in children, adolescents under 18 years, pregnant or nursing women, and patients with a known history of QTc interval prolongation or hypokalaemia. Concurrent use of Gatifloxacin with certain medications, such as digoxin or warfarin, necessitates close monitoring to avoid toxicity. Adherence to these guidelines is crucial to maximize the efficacy of Gatifloxacin while minimizing potential risks. 2
References:
[1] SUSAN J KEAM G M K Katherine F Croom. Gatifloxacin: a review of its use in the treatment of bacterial infections in the US.[J]. Drugs, 2005, 65 5. DOI:10.2165/00003495-200565050-00007.[2] Gatifloxacin[J]. Reactions Weekly, 2006, 64 1: 133-144. DOI:10.2165/00128415-200611080-00032.
Related articles And Qustion
Lastest Price from Gatifloxacin manufacturers
US $0.00/kg2025-11-19
- CAS:
- 112811-59-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $5.00-0.50/KG2025-06-13
- CAS:
- 112811-59-3
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg