Formamidine Acetate: Applications in Solar Cell Technology and its Preparation Method
General Description
Formamidine acetate significantly enhances the performance of Sn-based perovskite solar cells by improving crystallization quality and reducing trap density of states, which leads to higher efficiency. The combination of iodide-coordinated cation (FA+) and anion (AC-) facilitates the formation of high-quality FASnI3 films, achieving a champion power conversion efficiency of 9.96%. Additionally, devices with formamidine acetate show remarkable long-term stability, retaining 82% efficiency after 1500 hours of light aging. The preparation method involves a reflux reaction of trialkyl orthoformate, acetic acid, and ammonia, yielding high-purity formamidine acetate, which is crystallized using by-product alcohol as a solvent.

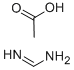
Figure 1. Formamidine acetate
Applications in Solar Cell Technology
Introduction to Formamidine Acetate in Solar Cell Technology
Formamidine acetate plays a crucial role in enhancing the performance of Sn-based perovskite solar cells, which are gaining significant attention in the field of photovoltaics. These solar cells are promising alternatives to traditional silicon-based technologies due to their potential for higher efficiency and lower production costs. However, a major challenge in developing these devices is the low crystallization quality of perovskite films. This challenge arises due to rapid crystallization processes that create a high trap density of states, leading to reduced device performance. The introduction of formamidine acetate addresses this issue by improving the crystallization process and, consequently, the overall quality of the perovskite films. 1
Mechanism of Action: Crystallization and Stabilization
The addition of formamidine acetate to the Sn-based perovskite solar cells significantly enhances the crystallization process. The presence of the iodide-coordinated cation, FA+, along with the crystallization-regulated anion, AC-, facilitates the formation of high-quality FASnI3 perovskite films. This combination leads to a reduction in defects within the crystal lattice, resulting in a lower trap density of states. As a consequence, the efficiency of the solar cells is notably improved. The research indicated that the champion power conversion efficiency achieved by FAAc-modified solar cells reached an impressive 9.96%. This milestone represents a significant advancement in the development of efficient and stable solar cell technologies. 1
Long-term Performance and Implications
The incorporation of formamidine acetate not only enhances the immediate performance of Sn-based perovskite solar cells but also contributes to their long-term stability. The devices modified with formamidine acetate demonstrated a remarkable retention of 82% of their initial power conversion efficiency during light aging tests over 1500 hours. This level of stability is particularly important for practical applications in renewable energy technologies. Overall, the findings associated with formamidine acetate provide valuable insights into the development of high-performance and durable Sn-based perovskite solar cells, paving the way for future innovations in photovoltaic technology. The strategic use of formamidine acetate can significantly influence the next generation of sustainable energy solutions. 1
Preparation Method
The preparation of formamidine acetate involves a straightforward process that utilizes the reaction of trialkyl orthoformate, acetic acid, and ammonia under reflux conditions. Initially, a mixture of trialkyl orthoformate—typically triethyl orthoformate—along with acetic acid and ammonia is heated. This reaction takes place at temperatures ranging from 115°C to 135°C, allowing the substances to react effectively and produce formamidine acetate. Upon completion of this step, the resultant mixture contains the desired formamidine acetate as well as by-product alcohol and other reaction intermediates. During this method, the efficiency of producing formamidine acetate is notable, achieving a purity level of 99.7% and an overall yield of 96%. This impressive yield makes the process not only efficient but also cost-effective. 2
Crystallization
Following the initial reaction, the crystallization step is essential for isolating pure formamidine acetate. In this phase, the alcoholic by-product, generated during the reaction, is utilized as a solvent to facilitate the crystallization of formamidine acetate. This approach not only minimizes waste but also enhances the purity of the final product. As the mixture cools, formamidine acetate crystallizes out of the solution, allowing for easy collection through filtration. The use of the by-product alcohol significantly contributes to reducing overall operational complexity while ensuring that the obtained formamidine acetate remains at a high purity level. Thus, the combination of efficient reaction conditions and strategic use of solvents results in a highly effective preparation method for formamidine acetate, achieving both purity and yield objectives successfully. 2
References:
[1] RUOYAO XU. Formamidine Acetate Induces Regulation of Crystallization and Stabilization in Sn-Based Perovskite Solar Cells[J]. ACS Applied Materials & Interfaces, 2021, 13 28: 32601-33744. DOI:10.1021/acsami.1c05097.See also
Lastest Price from Formamidine acetate manufacturers

US $1.00/KG2025-04-21
- CAS:
- 3473-63-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $10.00/kg2025-04-21
- CAS:
- 3473-63-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 ton