Fmoc-Trp(Boc)-OH Unveiled: A Comprehensive Exploration of its Role and Applications in Peptide Chemistry
Introduction
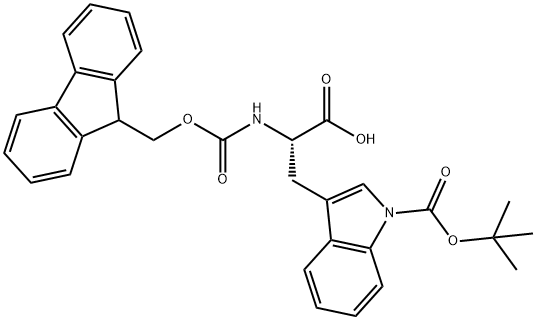
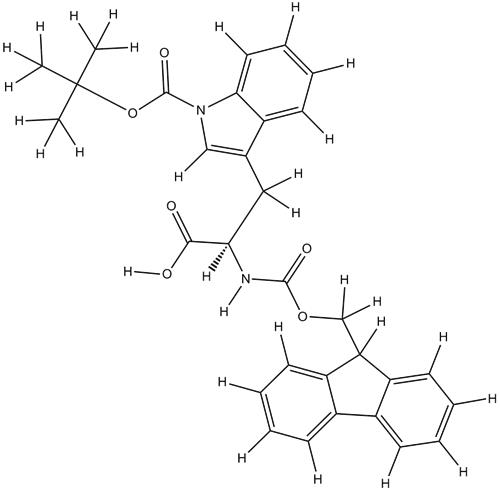
Fmoc-Trp(Boc)-OH is a protective group-modified amino acid derivative commonly used in biochemistry and organic synthesis. It is a prominent derivative in peptide chemistry, combining the benefits of both the Fmoc and Boc protection groups. This review delves into the characteristics, synthesis, and applications of Fmoc-Trp(Boc)-OH, offering a detailed examination of its role in peptide synthesis and drug development.1
Chemical Structure and Properties
Fmoc Group
The Fmoc (9-fluorenyl methoxycarbonyl) group is a widely used protecting group in solid-phase peptide synthesis. It provides stability and ease of removal under mild conditions, ensuring that the integrity of the peptide chain is maintained during synthesis.
Boc Group
The Boc (tert-butoxycarbonyl) group protects the indole nitrogen of tryptophan. This protection is crucial for preserving the tryptophan residue during peptide chain elongation and preventing unwanted reactions.
Combination
The synergy between the Fmoc and Boc groups in Fmoc-Trp(Boc)-OH provides a robust platform for constructing peptides with tryptophan residues. The Fmoc group aids in easy deprotection and coupling, while the Boc group ensures the stability of the tryptophan side chain.
Synthesis
Starting Materials
The synthesis of Fmoc-Trp(Boc)-OH typically begins with tryptophan, which is then selectively protected using the Boc group.
Fmoc Protection
The Fmoc group is introduced through a reaction with 9-fluorenylmethoxycarbonyl chloride, forming Fmoc-Trp(Boc)-OH. The synthesis requires careful control of reaction conditions to ensure selective protection.
Purification and Characterization
After synthesis, the compound is purified using techniques such as chromatography. Characterization is performed using NMR and mass spectrometry to confirm the structure and purity of Fmoc-Trp(Boc)-OH.2
Applications in Peptide Synthesis
Solid-Phase Peptide Synthesis (SPPS)
Fmoc-Trp(Boc)-OH is widely used in SPPS, where the Fmoc group allows for the sequential addition of amino acids to a growing peptide chain. The Boc group protects the tryptophan side chain during this process.
Peptide Mapping and Sequencing
The ability to include Fmoc-Trp(Boc)-OH in peptide sequences enables precise mapping and sequencing, particularly in studies involving tryptophan-rich peptides.
Custom Peptide Synthesis
This derivative is crucial for synthesizing custom peptides with specific tryptophan residues, which can be used to investigate protein interactions and functions.3
Role in Drug Development
Bioactive Peptides
Fmoc-Trp(Boc)-OH is instrumental in designing peptides with bioactive properties. Tryptophan's aromatic side chain can contribute to peptide stability and interactions with biological targets.
Therapeutic Agents
Peptides synthesized using Fmoc-Trp(Boc)-OH have potential applications as therapeutic agents. For example, they can be used in designing peptide-based drugs for treating diseases involving tryptophan metabolism.
Mimicking Protein Interactions
By incorporating tryptophan residues, researchers can create peptide analogs that mimic natural protein interactions, aiding in drug discovery and development.4
Advantages and Limitations
Advantages
The Fmoc-Trp(Boc)-OH derivative offers several benefits, including ease of removal of protecting groups, stability during synthesis, and the ability to incorporate tryptophan into peptides with high specificity.
Limitations
Despite its advantages, there are challenges associated with using Fmoc-Trp(Boc)-OH. These include the potential for steric hindrance and the need for careful control of reaction conditions to avoid side reactions.
Recent Advances and Future Directions
Innovations in Peptide Synthesis
Advances in peptide synthesis technologies continue to enhance the use of Fmoc-Trp(Boc)-OH, including developments in automated synthesis and high-throughput screening.
Exploring New Applications
Ongoing research aims to explore novel applications of Fmoc-Trp(Boc)-OH, including its role in developing new peptide-based therapeutics and materials.
Optimizing Synthetic Protocols
Future research will likely focus on optimizing synthetic protocols to improve yields and reduce costs associated with using Fmoc-Trp(Boc)-OH.5
Conclusion
Fmoc-Trp(Boc)-OH remains a cornerstone in peptide chemistry, offering versatility and precision in peptide synthesis and drug development. Its unique combination of protecting groups allows chemists to delve into complex peptide sequences with confidence, ultimately advancing the field of peptide-based research and therapeutic applications. As research progresses, Fmoc-Trp(Boc)-OH will undoubtedly continue to play a crucial role in shaping the future of peptide chemistry.
References:
[1] H. CHOI J V A. Comparison of methods for the Fmoc solid-phase synthesis and cleavage of a peptide containing both tryptophan and arginine[J]. Chemical Biology & Drug Design, 1993, 42 1: 1-96. DOI:10.1111/j.1399-3011.1993.tb00350.x.[2] THOMAS VORHERR W B ARNOLD TRZECIAK. Application of the allyloxycarbonyl protecting group for the indole of Trp in solid-phase peptide synthesis*[J]. Chemical Biology & Drug Design, 1996, 48 6: 495-578. DOI:10.1111/j.1399-3011.1996.tb00874.x.
[3] RAYMOND BEHRENDT P W Simon Huber. Preventing aspartimide formation in Fmoc SPPS of Asp-Gly containing peptides — practical aspects of new trialkylcarbinol based protecting groups[J]. Journal of Peptide Science, 2016, 22 2: 67-128. DOI:10.1002/psc.2844.
[4] TARU DUBE J J P Saurabh Mandal. Nanoparticles generated from a tryptophan derivative: physical characterization and anti-cancer drug delivery[J]. Amino Acids, 2017, 49 5. DOI:10.1007/s00726-017-2403-8.
[5] DR. ANAMIKA SHARMA. Understanding Tetrahydropyranyl as a Protecting Group in Peptide Chemistry[J]. ChemistryOpen, 2017, 6 2: 165-294. DOI:10.1002/open.201600156.
See also
Lastest Price from Fmoc-Trp(Boc)-OH manufacturers
US $0.00/kg2025-09-22
- CAS:
- 143824-78-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $0.00/Kg/Drum2025-04-21
- CAS:
- 143824-78-6
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 1000kg