Fluorescein: A Versatile Compound in Chemistry
Introduction
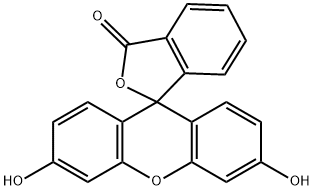
Fluorescein is a widely recognized and extensively used compound in the field of chemistry, known for its intense fluorescence and versatility across various applications. This synthetic organic compound is part of the xanthene dye family and has been pivotal in numerous scientific and medical fields due to its unique properties. In this article, we will explore the fundamental aspects of fluorescein, including its properties, composition, primary uses, and storage requirements.

Figure 1 Characteristics of Fluorescein
Properties of Fluorescein
Fluorescein is characterized by its vibrant green fluorescence when exposed to ultraviolet or blue light, making it a powerful tool in various analytical and diagnostic techniques. The chemical formula for fluorescein is C20H12O5, and its molecular structure consists of three benzene rings connected through a xanthene core. This structure allows fluorescein to absorb light in the UV-visible spectrum, typically around 494 nm, and emit light at a wavelength of approximately 521 nm. This emission of light is what gives fluorescein its distinctive fluorescence.
The compound has a molecular weight of 332.31 g/mol and appears as a reddish-orange powder in its pure form. It is soluble in alkaline solutions but shows poor solubility in water and organic solvents. Fluorescein’s pKa value, the pH at which it is half ionized, is approximately 6.4, which means it is more soluble in slightly alkaline conditions. The fluorescence of fluorescein is highly sensitive to pH changes, with its intensity increasing in alkaline environments. This sensitivity makes fluorescein a useful pH indicator in various chemical reactions and processes.
Major Components
Fluorescein is synthesized through the condensation of resorcinol with phthalic anhydride in the presence of a dehydrating agent, typically zinc chloride or concentrated sulfuric acid. The reaction produces fluorescein as the primary product, along with some byproducts that can be removed through purification processes.
In addition to the basic form of fluorescein, several derivatives and salts have been developed to enhance its properties for specific applications. One of the most common derivatives is fluorescein isothiocyanate (FITC), which is widely used in biochemistry for labeling proteins and other biomolecules. FITC retains the fluorescent properties of fluorescein but has an added isothiocyanate group that allows it to covalently bind to amines, enabling the tagging of various biomolecules.
Another important form is sodium fluorescein, which is the disodium salt of fluorescein and is highly soluble in water. Sodium fluorescein is extensively used in medical diagnostics, particularly in ophthalmology for fluorescein angiography, a technique used to visualize blood flow in the retina.
Applications of Fluorescein
The applications of fluorescein are vast and diverse, reflecting its importance across multiple fields of science and medicine.
Analytical Chemistry: In analytical chemistry, fluorescein is used as a tracer dye to monitor fluid flow in various systems. Due to its high sensitivity and distinct fluorescence, even small amounts of fluorescein can be detected, making it an ideal choice for tracing studies in hydrology, environmental science, and chemical engineering.
Medical Diagnostics: Fluorescein has a prominent role in medical diagnostics, particularly in ophthalmology. In fluorescein angiography, the dye is injected into the bloodstream, where it travels through the retinal blood vessels. The fluorescent properties of fluorescein allow for detailed imaging of the blood flow and the detection of abnormalities such as blockages, leaks, or abnormal blood vessel growth. This technique is crucial for diagnosing conditions like diabetic retinopathy and macular degeneration.
Biochemistry and Molecular Biology: In the field of biochemistry, fluorescein derivatives like FITC are commonly used to label proteins, nucleic acids, and other biomolecules for fluorescence microscopy, flow cytometry, and other fluorescence-based assays. These labeled molecules can be tracked and quantified with high precision, facilitating the study of cellular processes, protein interactions, and gene expression.
Forensic Science: Fluorescein is also utilized in forensic science for detecting latent blood stains. When sprayed on a surface and exposed to a UV light source, even trace amounts of blood can be made visible through fluorescence. This application is particularly valuable in crime scene investigations where the presence of blood may not be immediately apparent.
Industrial Applications: In industrial settings, fluorescein is employed to detect leaks in cooling systems, pipelines, and other fluid-containing systems. Its bright fluorescence makes it easy to spot even minor leaks, enabling timely maintenance and preventing potential hazards.
Storage and Handling
Proper storage and handling of fluorescein are crucial to maintain its stability and effectiveness. Fluorescein should be stored in a cool, dry place, away from direct sunlight and sources of moisture. The compound is relatively stable under normal conditions, but exposure to light and heat can cause it to degrade, leading to a reduction in its fluorescent properties.
When stored as a powder, fluorescein should be kept in tightly sealed containers to prevent it from absorbing moisture and degrading. Solutions of fluorescein, especially in alkaline media, should be prepared fresh or stored in the dark to preserve their fluorescence over time.
For laboratories and industrial settings where fluorescein is regularly used, it is essential to follow safety protocols, including the use of gloves, protective eyewear, and appropriate ventilation, to avoid inhalation or contact with the skin. In case of accidental exposure, affected areas should be rinsed thoroughly with water.
Conclusion
Fluorescein is a compound of significant importance in the field of chemistry, with applications spanning from analytical chemistry to medical diagnostics and industrial leak detection. Its unique fluorescent properties, coupled with its versatility in various forms, make it an invaluable tool for scientists and researchers. Understanding the properties, composition, and proper storage of fluorescein is essential for maximizing its effectiveness in these applications. As technology advances and new uses for fluorescein continue to emerge, it remains a cornerstone compound in both scientific research and practical applications.
[1] Sjöback R, Nygren J, Kubista M. Absorption and fluorescence properties of fluorescein[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 1995, 51(6): L7-L21.
[2] Martin M M, Lindqvist L. The pH dependence of fluorescein fluorescence[J]. Journal of Luminescence, 1975, 10(6): 381-390.
References:
[1] ROBERT SJ?BACK M K Jan Nygren. Absorption and fluorescence properties of fluorescein[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 1995, 51 6: L1-L24. DOI:10.1016/0584-8539(95)01421-P.[2] MONIQUE M. MARTIN L L. The pH dependence of fluorescein fluorescence[J]. Journal of Luminescence, 1975, 10 6: 347-423. DOI:10.1016/0022-2313(75)90003-4.
You may like
See also
Lastest Price from Fluorescein manufacturers

US $0.00/KG2025-04-21
- CAS:
- 2321-07-5
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $6.00/kg2025-04-21
- CAS:
- 2321-07-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000KG/Month