Fenofibrate: Pharmacological Activities, Pharmacokinetic Properties and Therapeutic Efficacy
General Description
Fenofibrate is a potent lipid-modifying agent that significantly improves lipid profiles and reduces cardiovascular risk through various pharmacological activities. Its active metabolite, fenofibric acid, activates peroxisome proliferator-activated receptor-alpha, enhancing lipolysis and promoting the clearance of triglyceride-rich lipoproteins. Clinical studies, including the pivotal FIELD trial, demonstrate that fenofibrate effectively reduces nonfatal myocardial infarction and improves overall cardiovascular outcomes, particularly in patients with dyslipidemia. Additionally, fenofibrate's pharmacokinetic properties ensure efficient absorption and elimination, supporting its long-term use while necessitating caution in specific populations, especially those with renal impairment.

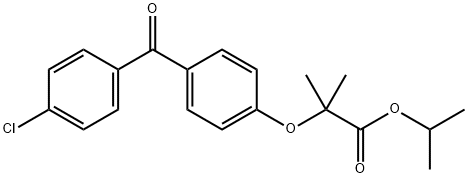
Figure 1. Fenofibrate
Pharmacological Activities
Effects on Lipids
Fenofibrate exerts significant pharmacological activities by modifying lipid profiles and influencing apolipoproteins through its active metabolite, fenofibric acid. The primary mechanism involves the activation of the nuclear transcription factor peroxisome proliferator-activated receptor-alpha (PPAR-alpha). When activated, PPAR-alpha forms a heterodimer with the retinoid X receptor, leading to the binding of this complex to specific peroxisome proliferator response elements. This interaction modulates gene expression related to lipid metabolism. Consequently, fenofibrate enhances lipolysis and promotes the plasma clearance of atherogenic triglyceride-rich lipoproteins by activating lipoprotein lipase and apolipoprotein A-V while simultaneously reducing apolipoprotein C-III levels, which inhibit lipoprotein lipase. Furthermore, fenofibrate facilitates the beta-oxidation of fatty acids, thereby decreasing the availability of free fatty acids necessary for triglyceride synthesis. Additionally, fenofibrate inhibits the synthesis of fatty acids via suppression of acetyl-CoA carboxylase and fatty acid synthase, contributing to a decrease in triglyceride levels. 1
Effects on Apolipoproteins and Lipoproteins
The pharmacological activities of fenofibrate extend to its effects on apolipoproteins, particularly in dyslipidemic conditions. Clinical studies have shown that fenofibrate significantly increases levels of apolipoprotein A-I and apolipoprotein A-II while reducing levels of non-high-density lipoprotein cholesterol, apolipoprotein C-III, and apolipoprotein B in patients with primary dyslipidemia, metabolic syndrome, or type 2 diabetes. These changes in apolipoprotein concentrations are essential as they directly influence cardiovascular risk profiles. Moreover, fenofibrate enhances the clearance of low-density lipoprotein cholesterol and alters its composition, resulting in larger and less dense particles that are less susceptible to oxidation. The activation of PPAR-alpha increases the synthesis of major high-density lipoprotein proteins, thus promoting cholesterol efflux from macrophages and improving overall lipid profiles. Fenofibrate's inhibitory action on cholesteryl ester transfer protein activity further supports its beneficial impact on increasing high-density lipoprotein cholesterol levels. Collectively, the pharmacological activities of fenofibrate are characterized by notable improvements in lipid parameters, contributing significantly to cardiovascular risk reduction. 1
Pharmacokinetic Properties
Absorption and Distribution
Fenofibrate is characterized by rapid absorption when taken orally, where it is metabolized by esterases into its active form, fenofibric acid. Following the administration of a micronized fenofibrate 200 milligram capsule, the mean plasma concentration of fenofibric acid is approximately 15 milligrams per milliliter, with the maximum plasma concentration reaching its peak about five hours after dosing. When evaluating the pharmacokinetic profile of fenofibrate delivered in a film-coated tablet form, a continuous dosing regimen of 160 milligrams daily yields a steady-state mean plasma concentration of 12.2 milligrams per milliliter within a timeframe of 3.5 hours. It is noteworthy that food intake significantly influences the absorption of fenofibrate; administering the film-coated tablet in a fed state enhances absorption by around 35% compared to fasting. In contrast, the nanoparticle formulation of fenofibrate at 145 milligrams displays a tmax ranging from 2 to 4 hours, remaining unaffected by food. Overall, fenofibrate and its metabolite, fenofibric acid, are predominantly bound to plasma albumin (over 99%), ensuring efficient distribution throughout the body's systemic circulation. 2
Elimination and Special Considerations
The elimination of fenofibrate occurs primarily through the urinary route, mainly in the form of fenofibric acid and its glucuronide conjugate. Within 24 hours post-administration, approximately 70% of the administered dose can be detected in the urine, demonstrating efficient excretion. The elimination half-life of fenofibric acid is roughly 20 hours, which is significant for understanding the dosing schedule. Importantly, fenofibrate does not accumulate in the body with repeated dosing, making it suitable for long-term use. However, special considerations must be taken for certain populations; for instance, no dosage adjustments are required for elderly patients without renal impairment. Yet, in cases of severe renal dysfunction, the use of fenofibrate is contraindicated. Additionally, while no significant alterations in pharmacokinetics are observed when combined with other medications, caution is advised with oral anticoagulants, as fenofibrate may potentiate their effects. Overall, understanding the pharmacokinetic properties of fenofibrate is crucial for optimizing its therapeutic use and minimizing potential adverse interactions. 2
Therapeutic Efficacy
Clinical Studies Overview
The therapeutic efficacy of fenofibrate has been evaluated through well-designed clinical studies, primarily focusing on adult populations. These studies typically included a drug-free or placebo run-in period to establish baseline measures, along with dietary control measures that participants maintained for the duration of the trial. Various formulations of fenofibrate, such as micronized capsules, tablets, and nanoparticle tablets, have been investigated. One of the most significant trials is the FIELD study, which primarily assessed cardiac outcomes in patients with type 2 diabetes mellitus and dyslipidemia. Patients in the FIELD trial were considered at increased risk for coronary heart disease but did not necessarily require lipid-lowering therapy, demonstrating the comprehensive applicability of fenofibrate in managing cardiovascular risks associated with metabolic disorders. 1
Clinical Outcomes and Comparisons
In the FIELD trial, while there was no significant difference in the incidence of coronary heart disease events between fenofibrate and placebo recipients, fenofibrate demonstrated a remarkable 24% relative reduction in nonfatal myocardial infarction events. Moreover, fenofibrate effectively lowered the risk of several macrovascular and microvascular complications when compared to placebo, achieving significant relative reductions in total cardiovascular disease events and revascularization procedures. The study highlighted that, over a five-year period, treating 70 patients with fenofibrate could potentially prevent one cardiovascular event. Subsequent analyses indicated that adjusting for concomitant lipid-lowering therapies improved the efficacy outcomes associated with fenofibrate. Additionally, stratified results showed that fenofibrate was particularly beneficial for patients presenting with marked hypertriglyceridemia, further supporting its role in tailored therapeutic approaches for dyslipidemia and associated cardiovascular risks. These findings illustrate the importance of fenofibrate in clinical settings aimed at optimal management of lipid profiles and cardiovascular health. 1
References:
[1] KATE MCKEAGE G M K. Fenofibrate: a review of its use in dyslipidaemia.[J]. Drugs, 2011, 71 14. DOI:10.2165/11208090-000000000-00000.[2] NICOLA TARANTINO. Fenofibrate and Dyslipidemia: Still a Place in Therapy?[J]. Drugs, 2018, 78 13. DOI:10.1007/s40265-018-0965-8.
Lastest Price from Fenofibrate manufacturers

US $0.00-0.00/kg2025-09-23
- CAS:
- 49562-28-9
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 1 tons

US $5.00-0.50/KG2025-05-15
- CAS:
- 49562-28-9
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg