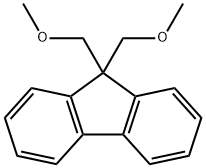
Exploring the Versatile World of 9,9-Bis(methoxymethyl)fluorene: Properties, Applications, and Handling
Introduction
In the dynamic world of chemical compounds, 9,9-Bis(methoxymethyl)fluorene stands out as a compound of increasing significance. Predominantly known for its versatile applications in the field of organic chemistry and material science, this compound has garnered attention for its unique properties and potential uses. Its distinctive molecular structure, characterized by the presence of methoxymethyl groups attached to the fluorene unit, renders it a compound of great interest among chemists and industry professionals. The relevance of 9,9-Bis(methoxymethyl)fluorene in modern chemical research and its growing importance in various industrial applications make it a noteworthy topic for discussion in the chemical community[1].

Figure 1 Characteristics of 9,9-BIS(METHOXYMETHYL)FLUORENE
Chemical Properties and Structure
Understanding the chemical properties and structure of 9,9-Bis(methoxymethyl)fluorene is pivotal in appreciating its potential and versatility. This compound is a derivative of fluorene, a polycyclic aromatic hydrocarbon, distinguished by its two methoxymethyl groups positioned at the 9th carbon of the fluorene structure. This structural alteration significantly impacts both the physical and chemical characteristics of the compound.
Molecular Structure
The molecular geometry of 9,9-Bis(methoxymethyl)fluorene plays a critical role in its reactivity and interaction with other substances. Its planar structure, typical of aromatic compounds, facilitates π-π interactions, making it an ideal candidate for certain types of organic reactions. Additionally, the methoxymethyl groups contribute to its solubility in organic solvents, expanding its utility in various chemical processes.
Physical Properties
9,9-Bis(methoxymethyl)fluorene is distinguished by its solid state at room temperature, and its melting and boiling points are indicative of strong intermolecular forces, typical of aromatic compounds. Its solubility profile includes good solubility in common organic solvents, which is crucial for its applications in solution-based processes.
Chemical Reactivity
In terms of reactivity, 9,9-Bis(methoxymethyl)fluorene exhibits stability under standard conditions but can participate in a variety of chemical reactions. Its reactivity is significantly influenced by the presence of the methoxymethyl groups, which can transform specific conditions, thereby altering the compound’s reactivity profile.
Mechanism of Action
The mechanism of action of 9,9-Bis(methoxymethyl)fluorene is a fascinating aspect that reveals much about its applications and importance in chemical processes. This section delves into the molecular interactions and reactions this compound undergoes, providing insight into its behavior and functionality.
Molecular Interactions
At a molecular level, 9,9-Bis(methoxymethyl)fluorene exhibits unique interactions due to its aromatic nature and the presence of methoxymethyl groups. These interactions include π-π stacking, which is fundamental in many organic reactions, particularly in the formation of supramolecular structures. The electron-rich nature of the fluorene core also allows it to participate in electron donor-acceptor interactions, contributing to its utility in materials science.
Reactivity with Other Substances
The reactivity of 9,9-Bis(methoxymethyl)fluorene with other substances varies depending on the environmental conditions and the nature of the reacting species. For instance, it can undergo electrophilic aromatic substitution, given its aromatic structure. Moreover, the methoxymethyl groups can be involved in reactions like hydrolysis under certain conditions, transforming the compound and altering its properties.
Role in Synthesis and Catalysis
A notable aspect of its mechanism of action is its role in synthesis and catalysis. 9,9-Bis(methoxymethyl)fluorene can act as a building block in the synthesis of complex organic molecules. Additionally, its unique structure can facilitate its role as a ligand in catalytic processes, where it can bind to metal centers and influence the course of catalytic reactions.
Applications
The diverse applications of 9,9-Bis(methoxymethyl)fluorene span across various domains, underscoring its significance in both industrial and research settings. This compound's unique properties lend it to numerous practical and innovative uses[2].
In Organic Synthesis
A primary application of 9,9-Bis(methoxymethyl)fluorene is in the field of organic synthesis. It serves as a crucial intermediate in the synthesis of complex organic molecules, especially in pharmaceuticals and agrochemicals. Its ability to undergo a range of reactions makes it a versatile reagent in creating various molecular structures.
Materials Science
In materials science, 9,9-Bis(methoxymethyl)fluorene finds applications in the development of new materials. Its structural properties are beneficial in creating polymers and composite materials, particularly in electronics and photonics.
Catalysis
Another significant application is in catalysis. The unique structure of 9,9-Bis(methoxymethyl)fluorene allows it to function as a ligand in metal-catalyzed reactions. This role is pivotal in enhancing the efficiency and selectivity of various catalytic processes, crucial in both industrial and environmental applications.
Storage and Handling
Proper storage and handling of 9,9-Bis(methoxymethyl)fluorene is crucial for maintaining its integrity and ensuring safety in the workplace. Given its chemical nature, specific protocols must be followed to prevent degradation and minimize risks.
Storage Conditions
Temperature Control
9,9-Bis(methoxymethyl)fluorene should be stored in a cool, dry place, away from direct sunlight. Maintaining a stable temperature helps in preserving its chemical structure and preventing any unwanted reactions.
Moisture Prevention
It is essential to store this compound in an airtight container to protect it from moisture, as humidity can lead to hydrolysis of the methoxymethyl groups.
Light Sensitivity
Exposure to light, especially UV light, can affect the compound. Hence, storing it in amber-colored or opaque containers can prevent photodegradation.
Handling Precautions
Personal Protective Equipment (PPE)
When handling 9,9-Bis(methoxymethyl)fluorene, appropriate PPE such as gloves, safety glasses, and lab coats should be worn to prevent skin contact and eye exposure.
Ventilation
Work with this compound should be conducted in a well-ventilated area, preferably under a fume hood, to avoid inhalation of any vapors or dust.
Waste Disposal
Any waste containing 9,9-Bis(methoxymethyl)fluorene should be disposed of according to local regulations for hazardous waste, as improper disposal can pose environmental risks.
Safety Measures
In case of skin contact or inhalation, immediate medical attention should be sought. Material safety data sheets (MSDS) must be readily available in the storage and handling areas for quick reference in case of emergencies.
References
[1]Brambilla L, Zerbi G, Piemontesi F, et al. Structure of Donor Molecule 9, 9-Bis (Methoxymethyl)-Fluorene in Ziegler-Natta Catalyst by Infrared Spectroscopy and Quantum Chemical Calculation[J]. The Journal of Physical Chemistry C, 2010, 114(26): 11475-11484.
[2]Zhong C, Gao M, Mao B. Effect of Et3Al and 9, 9‐Bis (methoxymethyl) fluorine on Propylene Polymerization at High Temperature with TiCl4/MgCl2 Catalysts[J]. Macromolecular Chemistry and Physics, 2005, 206(3): 404-409.
Related articles And Qustion

US $0.00-0.00/g2024-12-24
- CAS:
- 182121-12-6
- Min. Order:
- 1g
- Purity:
- 99.99%
- Supply Ability:
- 20 tons
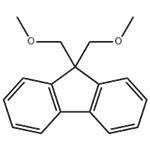
US $35.00-1.40/kg2024-03-27
- CAS:
- 182121-12-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available