Exploring the Multifaceted World of 4-Aminohippuric Acid: Synthesis, Applications, and Clinical Insights
Introduce
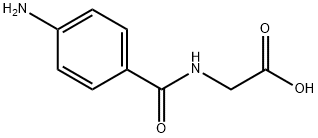
4-Aminohippuric acid, commonly known as PAH, is a compound of significant interest in the fields of chemistry and medicine. Renowned for its role in renal function tests, PAH's utility extends beyond mere diagnostic applications, touching various aspects of biochemical research and therapeutic interventions. Its diagnostic prowess in nephrology is unmatched, offering critical insights into kidney health and function.
With its unique structure, PAH serves as a pivotal tool in nephrology, aiding in the assessment of renal plasma flow and glomerular filtration rate. Beyond medical diagnostics, it plays a crucial role in the study of renal physiology and pathophysiology, enhancing our understanding of kidney diseases. Its significance, however, is not confined to the medical domain; in chemistry, 4-aminohippuric acid provides insights into compound interaction dynamics and metabolic pathways, showcasing its versatility and critical role in scientific advancements. This compound's multifaceted applications highlight its importance in advancing both clinical practice and biochemical research, making it indispensable in the scientific community.

Figure 1 Characteristics of 4-Aminohippuric acid
Synthesis method
The synthesis of 4-aminohippuric acid is a testament to the ingenuity in chemical engineering and organic chemistry, showcasing a sophisticated blend of reactivity and precision. This process typically begins with the amino acid glycine, which undergoes a series of reactions to introduce the hippuric structure, culminating in the attachment of the amino group that defines 4-aminohippuric acid.
Starting Material
The journey of synthesizing 4-aminohippuric acid commences with benzoyl chloride, which reacts with glycine in a controlled environment. This reaction is a classic example of acylation, where the benzoyl group is transferred to the amino acid, forming hippuric acid as an intermediate product.
Nitration
The next crucial step involves the nitration of hippuric acid. This process introduces a nitro group into the benzene ring of the hippuric acid molecule. Employing a mixture of nitric and sulfuric acids, the nitration step must be meticulously controlled to ensure the correct position of the nitro group, aiming for the para position relative to the existing carbonyl group.
Reduction
Following nitration, the nitro group attached to the benzene ring is reduced to an amino group. This reduction is typically achieved using a variety of agents, with hydrogen in the presence of a catalyst being a common choice. The reduction transforms the nitro group into an amine, yielding 4-aminohippuric acid.
Purification
The final stage in the synthesis of 4-aminohippuric acid is its purification. This step is crucial to remove any unreacted starting materials, by-products, or impurities. Techniques such as recrystallization, chromatography, or distillation are employed to obtain pure 4-aminohippuric acid, suitable for various applications.
The synthesis of 4-aminohippuric acid is a fine illustration of the principles of organic synthesis, where each step is designed to introduce or modify specific functional groups in a controlled and predictable manner. The challenges in this synthesis often revolve around controlling the reaction conditions to ensure the desired product is obtained with high yield and purity. Innovations and optimizations in the synthesis process continue to evolve, reflecting the dynamic nature of chemical research and its application to real-world problems.
Application
4-Aminohippuric acid (PAH) plays a pivotal role in various scientific and medical domains, showcasing its versatility and importance. Its applications range from renal function diagnostics to being a tool in biochemical research, illustrating the compound's multifaceted utility[2].
Renal Function Assessment
PAH is extensively used in the medical field to assess renal plasma flow and kidney function. The PAH clearance test is a classic and critical diagnostic tool in nephrology, aiding in the evaluation of the effective renal plasma flow (ERPF). By measuring the rate at which PAH is cleared from the plasma, healthcare professionals can obtain valuable insights into the functional capacity of the kidneys, particularly the perfusion of the renal cortex.
Research Tool in Pharmacokinetics
In the realm of pharmacokinetics, 4-aminohippuric acid serves as a vital tool for studying drug excretion and renal clearance mechanisms. Its use helps in understanding how substances are filtered and secreted by the kidneys, providing a deeper comprehension of drug interactions and the renal elimination of various compounds.
Biochemical Studies
PAH is utilized in biochemical research to study the transport mechanisms of organic anions in the kidney. Its ability to be actively secreted by renal tubular cells makes it an excellent probe for investigating the dynamics of renal tubular transport systems, offering insights into the molecular basis of substance exchange and the role of transporters in renal physiology.
Diagnostic Agent
Beyond its use in research, PAH is employed as a diagnostic agent in certain medical tests, including the measurement of renal plasma flow. Its properties allow for precise and reliable assessment, contributing to the accurate diagnosis and monitoring of renal health.
Educational Purposes
In academic settings, the synthesis and application of 4-aminohippuric acid are often included in chemistry and pharmacology curricula. It serves as an example of compound synthesis and its practical applications in medicine, helping students understand the link between chemical synthesis and clinical application.
Usage and dosage
The correct usage and dosage of 4-aminohippuric acid (PAH) are crucial for its effectiveness, particularly in medical diagnostics and research applications. Ensuring the appropriate administration of PAH is essential for obtaining accurate results and minimizing potential risks.
Diagnostic Use in Renal Function Tests
In the clinical setting, particularly for the PAH clearance test, the compound is administered intravenously. The typical dose for an adult patient is adjusted to body surface area, usually starting at about 10 mg per kg of body weight. The infusion is maintained at a rate that ensures steady plasma levels, which is critical for accurate measurement of renal plasma flow. During the test, blood and urine samples are collected at specific intervals to measure the concentration of PAH, allowing the calculation of renal plasma flow.
Research Applications
In research studies, especially those involving animal models, the dosage of PAH may vary based on the specific objectives and the species being studied. The administration route can be intravenous, intraperitoneal, or oral, depending on the experimental design. Researchers must calculate the dose meticulously, considering factors such as the animal's weight, the route of administration, and the desired plasma concentration.
Adverse reactions
While 4-aminohippuric acid (PAH) is generally considered safe for use in medical diagnostics and research, awareness of its potential adverse reactions is essential for healthcare providers and researchers. Monitoring for these reactions ensures the safety of patients and subjects involved in PAH-related procedures.
Allergic Reactions
Like any compound introduced into the body, PAH has the potential to elicit allergic reactions in some individuals. Symptoms can range from mild (rashes, itching) to severe (anaphylaxis). Immediate medical attention is necessary if signs of a severe allergic reaction occur.
Local Reactions at Injection Site
In the case of intravenous administration, patients may experience local reactions at the injection site, such as pain, redness, or swelling. These reactions are usually transient and resolve without intervention.
Systemic Effects
Although rare, systemic effects can occur, particularly at higher doses. These may include nausea, dizziness, or headaches. It is crucial to monitor patients for any signs of systemic adverse effects during and after the administration of PAH.
Renal Effects
Given that PAH is used primarily for assessing renal function, patients with pre-existing renal impairment require careful monitoring. While PAH is instrumental in diagnosing renal issues, its administration in compromised renal function must be approached with caution to avoid exacerbating the condition.
Interactions with Other Medications
PAH's interaction with other medications is an important consideration. It can potentially interfere with the renal clearance of other drugs, leading to altered drug levels in the blood. Healthcare providers should review the patient's medication history to identify possible drug interactions.
References
[1]Dobson, Allison J., and Roger E. Gerkin. "Hydrogen bonding in 4-aminohippuric acid."Acta Crystallographica Section C: Crystal Structure Communications55.2 (1999): 206-208.
[2]Mohadesi, Alireza, et al. "4-Aminohippuric acid-functionalized carbon nanotubes for strip** voltammetric determination of copper (II) ions."Electrochemistry84.3 (2016): 138-142.
Related articles And Qustion
Lastest Price from 4-Aminohippuric acid manufacturers

US $0.00/kg2025-08-21
- CAS:
- 61-78-9
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00-0.00/KG2025-04-04
- CAS:
- 61-78-9
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton