Ethylenediaminetetraacetic acid: Application, toxicity and environmental fate
General description
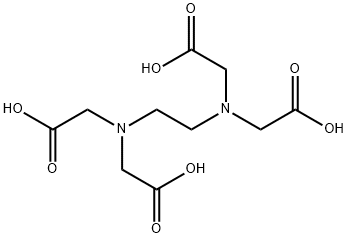
Ethylenediaminetetraacetic acid (EDTA) is an aminopolycarboxylic acid, and widely used to bind to iron and calcium ions. It binds these ions as a hexadentate ("six-toothed") chelating agent. EDTA is produced as several salts, notably disodium EDTA, sodium calcium edetate, and tetrasodium EDTA. In industry, Ethylenediaminetetraacetic acid is mainly used to sequester metal ions in aqueous solution. In the textile industry, it prevents metal ion impurities from modifying colours of dyed products. In the pulp and paper industry, EDTA inhibits the ability of metal ions, especially Mn2+, from catalysing the disproportionation of hydrogen peroxide, which is used in chlorine-free bleaching. In a similar manner, Ethylenediaminetetraacetic acid is added to some food as a preservative or stabiliser to prevent catalytic oxidative decolouration, which is catalysed by metal ions. In soft drinks containing ascorbic acid and sodium benzoate, EDTA mitigates formation of benzene (a carcinogen). Its appearance is as follows:

Application
Sodium calcium edetate, an EDTA derivative, is used to bind metal ions in the practice of chelation therapy, such as for treating mercury and lead poisoning. It is used in a similar manner to remove excess iron from the body. This therapy is used to treat the complication of repeated blood transfusions, as would be applied to treat thalassaemia. Ethylenediaminetetraacetic acid is used extensively in the analysis of blood. It is an anticoagulant for blood samples for CBC/FBCs, where the Ethylenediaminetetraacetic acid chelates the calcium present in the blood specimen, arresting the coagulation process and preserving blood cell morphology.[1] Ethylenediaminetetraacetic acid is a slime dispersant, and has been found to be highly effective in reducing bacterial growth during implantation of intraocular lenses (IOLs).[2]
In shampoos, cleaners, and other personal care products, Ethylenediaminetetraacetic acid salts are used as a sequestering agent to improve their stability in air.[3] In the laboratory, EDTA is widely used for scavenging metal ions: In biochemistry and molecular biology, ion depletion is commonly used to deactivate metal-dependent enzymes, either as an assay for their reactivity or to suppress damage to DNA, proteins, and polysaccharides. Ethylenediaminetetraacetic acid also acts as a selective inhibitor against dNTP hydrolyzing enzymes (Taq polymerase, dUTPase, MutT), liver arginase and horseradish peroxidase[4] independently of metal ion chelation.
Toxicity
Ethylenediaminetetraacetic acid exhibits low acute toxicity with LD50 (rat) of 2.0 g/kg to 2.2 g/kg. It has been found to be both cytotoxic and weakly genotoxic in laboratory animals. Oral exposures have been noted to cause reproductive and developmental effects.[3] The same study [3] also found that both dermal exposure to EDTA in most cosmetic formulations and inhalation exposure to EDTA in aerosolised cosmetic formulations would produce exposure levels below those seen to be toxic in oral dosing studies.
Environmental fate
Ethylenediaminetetraacetic acid is in such widespread use that questions have been raised whether it is a persistent organic pollutant. While EDTA serves many positive functions in different industrial, pharmaceutical and other avenues, the longevity of EDTA can pose serious issues in the environment. The degradation of Ethylenediaminetetraacetic acid is slow. It mainly occurs abiotically in the presence of sunlight.[5]
The most important process for the elimination of EDTA from surface waters is direct photolysis at wavelengths below 400 nm. Depending on the light conditions, the photolysis half-lives of iron(III) Ethylenediaminetetraacetic acid in surface waters can range as low as 11.3 minutes up to more than 100 hours.[6] Degradation of FeEDTA, but not EDTA itself, produces iron complexes of the triacetate (ED3A), diacetate (EDDA), and monoacetate (EDMA) – 92% of EDDA and EDMA biodegrades in 20 hours while ED3A displays significantly higher resistance. Many environmentally-abundant EDTA species (such as Mg2+ and Ca2+) are more persistent.
In many industrial wastewater treatment plants, Ethylenediaminetetraacetic acid elimination can be achieved at about 80% using microorganisms. Resulting byproducts are ED3A and iminodiacetic acid (IDA) – suggesting that both the backbone and acetyl groups were attacked. Some microorganisms have even been discovered to form nitrates out of Ethylenediaminetetraacetic acid, but they function optimally at moderately alkaline conditions of pH 9.0–9.5.
References
[1]Banfi, G; Salvagno, G. L; Lippi, G (2007). The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clinical Chemistry and Laboratory Medicine. 45 (5): 565–76.
[2]Kadry, A. A.; Fouda, S. I.; Shibl, A. M.; Abu El-Asrar, A. A. (2009). Impact of slime dispersants and anti-adhesives on in vitro biofilm formation of Staphylococcus epidermidis on intraocular lenses and on antibiotic activities. Journal of Antimicrobial Chemotherapy. 63 (3): 480–4.
[3]Lanigan, R. S.; Yamarik, T. A. (2002). Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. International Journal of Toxicology. 21 Suppl. 2 (5): 95–142.
[4]Bhattacharyya, D K; Adak, S; Bandyopadhyay, U; Banerjee, R K (1994-03-01). Mechanism of inhibition of horseradish peroxidase-catalysed iodide oxidation by EDTA. Biochemical Journal. 298 (Pt 2): 281–288.
[5]Bucheli-Witschel, M.; Egli, T. (2001), DAB: Environmental Fate and Microbial Degradation of Aminopolycarboxylic Acids, FEMS Microbiology Reviews, 25 (1): 69–106
[6]Frank, R.; Rau, H. (1989). Photochemical transformation in aqueous solution and possible environmental fate of Ethylenediaminetetraacetatic acid (EDTA). Ecotoxicology and Environmental Safety. 19 (1): 55–63.
Related articles And Qustion
See also
Lastest Price from Ethylenediaminetetraacetic acid manufacturers

US $55.00-47.00/kg2025-11-03
- CAS:
- 60-00-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100tons

US $50.00-10.00/kg2025-09-02
- CAS:
- 60-00-4
- Min. Order:
- 1kg
- Purity:
- 99%,Electronic grade(Single metal impurity≤ 100ppb) or pharmaceutical grade
- Supply Ability:
- 100kg