Ethyl lactate: properties, synthesis and biosynthetic pathway
General Description
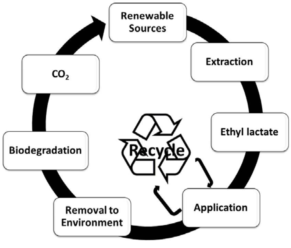
Ethyl lactate, a sustainable solvent with the formula C5H10O3, is produced from lactic acid and ethanol. It's a biodegradable, low-toxicity liquid, miscible with water and organic solvents, and has a wide liquid range (melting point -25°C, boiling point 154°C), making it suitable for various applications including paints and cleaners. Its production can be optimized using Zr-based catalysts or process intensification techniques like reactive distillation. Additionally, biosynthesis in modified Saccharomyces cerevisiae involving key enzymes can increase ethyl lactate yields, serving as a raw material in flavorings and fragrances.

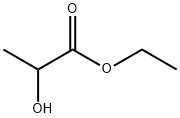
Figure 1. Ethyl lactate
Properties
Ethyl lactate, also known as lactic acid ethyl ester, is a monobasic ester formed from lactic acid and ethanol, commonly used as a solvent. It is a clear, colorless liquid with a characteristic mild odor and a slight fruity taste. Its molecular formula is C5H10O3, and it has a molecular weight of 118.13 g/mol. This compound is readily biodegradable and is considered environmentally friendly due to its low toxicity and low potential for bioaccumulation. Ethyl lactate has a boiling point of approximately 154°C (309°F) and a melting point of -25°C, which indicates that it remains a liquid over a wide temperature range, making it suitable for various applications. It is miscible with most organic solvents and water, which allows it to be used in diverse chemical processes. Its solvency power makes it effective in formulations for paints, coatings, inks, and cleaners. Moreover, ethyl lactate has a relatively high flash point (46°C or 115°F), which contributes to its safety in handling compared to more volatile solvents. In summary, ethyl lactate's favorable properties such as biodegradability, low toxicity, broad liquid range, and good solvency, coupled with its environmental compatibility, make it a valuable solvent in industrial applications. 1
Synthesis
Ethyl lactate is a green solvent derived from renewable resources, offering an eco-friendly alternative to petroleum-based volatile organic compounds (VOCs). Traditionally, it is synthesized through the esterification of lactic acid with ethanol. A study explores two distinct strategies for producing ethyl lactate in a more sustainable and cost-effective manner. The first approach utilizes eco-friendly Zr-based catalysts, specifically basic zirconium carbonate, which has demonstrated superior performance in converting dihydroxyacetone (a biomass derivative) to ethyl lactate. Operating at 140 °C and under 1.0 MPa N2 pressure for 4 hours, this catalyst achieved a full conversion of dihydroxyacetone with an 85.3% yield of ethyl lactate. The efficacy of basic zirconium carbonate is attributed to the synergistic effects of its acidic and basic active sites, and it also exhibits excellent thermal stability and reusability, maintaining its activity over multiple runs. The second strategy involves process intensification through reactive distillation, which combines the reaction and separation processes, enhancing energy efficiency and space-time yield. An optimized serial setup of reactors followed by distillation steps has been identified as the most energy-efficient system. This configuration provides similar benefits to simultaneous reactive distillation but with greater flexibility and ease of scale-up, making it a favorable option for industrial production of ethyl lactate. 2,3
Biosynthetic pathway
Ethyl lactate, a key flavor component in baijiu and a raw material in fragrance production, can be biosynthesized in engineered Saccharomyces cerevisiae. The pathway commences with the introduction of propionyl-CoA transferase, which catalyzes the formation of propionyl-CoA, a precursor for ethyl lactate synthesis. Concurrently, alcohol acyltransferase facilitates the esterification of ethanol with acyl-CoA derivatives to produce ethyl esters like ethyl lactate. To enhance production, the copy number of genes encoding these enzymes, Pctcp and AeAT9, is increased, leading to higher enzyme concentrations and thus greater ethyl lactate yields. Further optimization involves genetic modifications to reduce pyruvate transport into mitochondria by knocking out porin and mitochondrial pyruvate carrier genes. This strategy redirects pyruvate towards cytosolic pathways, favoring ethyl lactate synthesis and culminating in a significant increase in its production. 4
Reference
1. Ethyl lactate. National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 7344.
2. Shi J, Li F, Zhang J, Li N, Wang X, Zhang X, Liu Y. One-pot conversion of dihydroxyacetone into ethyl lactate by Zr-based catalysts. RSC Adv. 2021 Mar 15;11(18):10935-10940.
3. Stipsitz P, Mandl M, Harasek M. Ethyl lactate production by reactive distillation - optimization of reaction kinetics and energy efficiency. Open Res Eur. 2021 Sep 23;1:82.
4. Ren JY, Liu G, Chen YF, Jiang S, Ma YR, Zheng P, Guo XW, Xiao DG. Enhanced Production of Ethyl Lactate in Saccharomyces cerevisiae by Genetic Modification. J Agric Food Chem. 2020 Nov 25;68(47):13863-13870.
Related articles And Qustion
See also
Lastest Price from Ethyl lactate manufacturers

US $10.70/KG2025-06-10
- CAS:
- 97-64-3
- Min. Order:
- 10KG
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $10.00/KG2025-04-21
- CAS:
- 97-64-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt