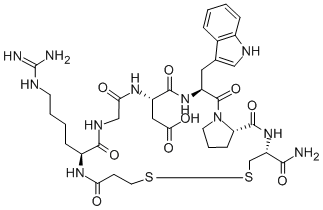
Eptifibatide acetate: Chemical property and process optimization
Introduction
The cyclic heptapeptide eptifibatide (N6-(aminoiminomethyl)-N2-(3-mercapto-1-oxopropyl)-Llysylglycyl-L-α-aspartyl-L-tryptophyl-L-prolyl-L-cysteinamide, cyclic (1→6)-disulfide), is a reversible inhibitor of platelet aggregation. This potent and specific glycoprotein IIb/IIIa receptor antagonist is mainly indicated for the treatment of acute coronary syndrome, including unstable angina or non-Q-wave myocardial infarction and patients undergoing primary percutaneous coronary intervention.
Chemical property
Eptifibatide acetate is a white or white-off powder, soluble in water and freely soluble in 1% acetic acid in water. Its empirical formula is C35H49N11O9S2, with a molecular weight of 831.96. Eptifibatide acetate was approved by the FDA in 1998[1].
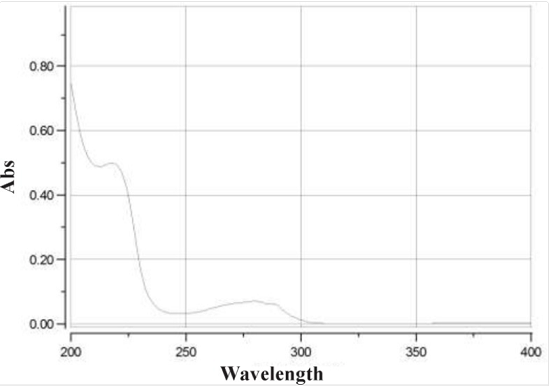
UV spectrum of eptifibatide acetate solution showed maximum absorbance at 219 and 275 nm. The assay chromatograms at 219 and 275 nm are presented in below. The wavelength of 275 nm was selected as suitable detection wavelength because of clear flat baseline and being prevented from interference coming from TFA along with symmetrical response peak.
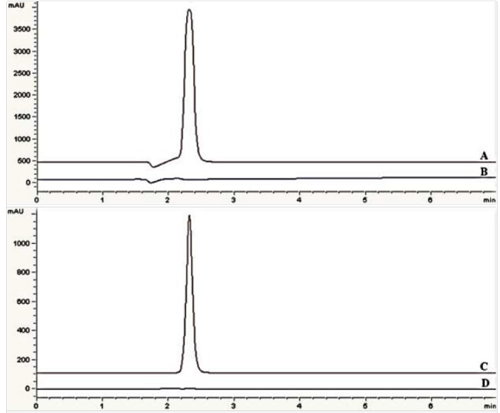
Chromatograms of: (A) standard solution, 0.75 mg/mL eptifibatide acetate in 219 nm (B) blank sample (deionized water) in 219 nm (C) standard solution, 0.75 mg/mL eptifibatide acetate in 275 nm (D) blank sample (deionized water) in 275 nm
laboratory process optimization
D’Ercole et al. report the laboratory process optimization (1−5 mmol) of the heterodetic cyclopeptide API eptifibatide acetate in five steps: (1) eptifibatide linear precursor automated MW-assisted solid-phase synthesis (Liberty Blue, CEM, Charlotte, NC, U.S.A, step 1); (2) cleavage from the resin and amino acid side-chain deprotection (step 2); (3) in solution disulfide-bond formation (step 3); (4) purification by flash column chromatography (step 4), followed by (5) ionexchange solid-phase extraction (step 5)[2].
References
[1] Maryam Bavand Savadkouhi. “RP-HPLC Method Development and Validation for Determination of Eptifibatide Acetate in Bulk Drug Substance and Pharmaceutical Dosage Forms.” Iranian Journal of Pharmaceutical Research 16 2 (2017): 490–497.
[2] Annunziata D’Ercole. “An Optimized Safe Process from Bench to Pilot cGMP Production of API Eptifibatide Using a Multigram-Scale Microwave-Assisted Solid-Phase Peptide Synthesizer.” Organic Process Research & Development 25 12 (2021): 2754–2771.
Lastest Price from Eptifibatide Acetate manufacturers

US $5.00/Box2025-04-27
- CAS:
- 148031-34-9
- Min. Order:
- 1Box
- Purity:
- 99.99%
- Supply Ability:
- 20000 boxes

US $0.00/g2025-04-21
- CAS:
- 148031-34-9
- Min. Order:
- 1g
- Purity:
- 98%min
- Supply Ability:
- 1000g