Emtricitabine---A potent, synthetic nucleoside reverse transcriptase inhibitor.
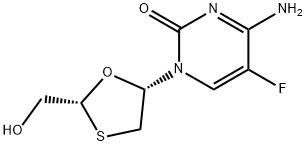
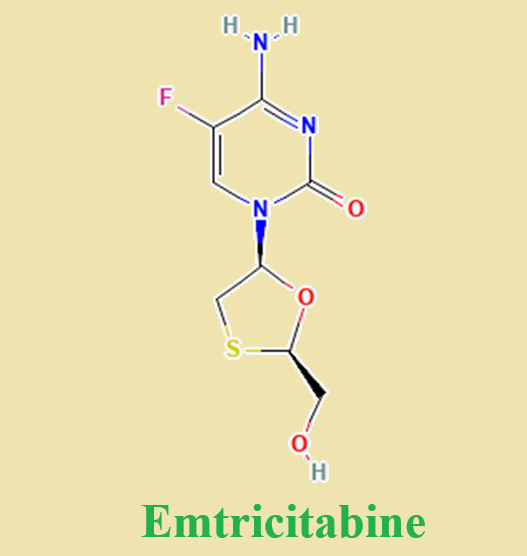
Emtricitabine (also known as fluorthiocytidine or FTC) is the negative enantiomer of a thio analog of dideoxycytidine with a fluorine in the 5 position. Emtricitabine is a potent, synthetic nucleoside reverse transcriptase inhibitor with selective activity against HIV-1 and -2 and hepatitis B virus (HBV). The drug was initially synthesized by Triangle Pharmaceuticals, Inc. (brand name Coviracils) and was subsequently marketed by Gilead Sciences, Inc. as Emtrivas in 2004, and more recently under the trade name FTCs. A tablet containing emtricitabine co-formulated with tenofovir is marketed as Truvadas and a tablet co-formulated with efavirenz and tenofovir is marketed as Atriplas; both of which are also marketed by Gilead Sciences.
Emtricitabine is approved for the treatment of HIV-1 infection in a number of countries, including Australia, Europe, and the USA. The urgent need for once-daily dosing in antiretroviral therapy has expedited the registration of emtricitabine, based on the association between adherence, treatment outcome, and patient preferences reported in multiple studies by not only Gilead Sciences, but a number of other independent research groups as well. The chemical structure of emtricitabine differs from that of lamivudine in that it has a fluorine atom in the 5u position of the pyrimidine ring, as opposed to lamivudine, which has a hydrogen atom. Otherwise the b-L structural configuration of emtricitabine [(–)-FTC] is similar to lamivudine [(–)-3TC]. Emtricitabine differs from other cytidine analogs because of the fluorine in the 5u position . Emtricitabine is available as capsule for oral administration containing 200 mg of drug, and as an oral solution at a concentration of 10 mg/ml.
MECHANISM OF DRUG ACTION
Like other nucleoside analogs, emtricitabine is a prodrug that must be triphosphorylated by intracellular enzymes into its active form, emtricitabine 5u-triphosphate. Phosphorylation is mediated by host cell enzymes. The triphosphate inhibits the activity of HIV and HBV reverse transcriptases by competing with the endogenous substrate for incorporation into the growing DNA chain. Incorporation of emtricitabine 5u-triphosphate into the viral DNA causes chain termination, thereby irreversibly inhibiting viral replication. The fact that emtricitabine 5u-triphosphate is incorporated more efficiently than lamivudine 5u-triphosphate during RNA-dependent DNA synthesis it is believed to be the contributing factor for the greater potency of emtricitabine compared with lamivudine.
CLINICAL USES
Emtricitabine is currently indicated, in combination with other antiretroviral agents, for the treatment of HIV-1 and HIV-2 infection in adults aged Z18 years and in children aged three months to 17 years who are naive to antiretroviral medication or who are changing therapy, where their HIV strain remains susceptible to emtricitibine. This indication is supported by clinical trials conducted with previously untreated and antiretroviral-experienced individuals. Additive to synergistic effects are observed when emtricitabine is added to nucleoside reverse transcriptase inhibitors, NNRTIs, and protease inhibitors. Emtricitabine and lamivudine, although potent nucleoside analog reverse transcriptase inhibitors in their own right, are generally combined with other nucleoside or nucleotide analogs, for examples, zidovudine, stavudine, didanosine, abacavir, or tenofovir. The principal reason for this treatment strategy is that the genetic barrier to resistance with emtricitibine and lamivudine is low, as a single M184V mutation results in a Z200-fold decrease in susceptibility, whereas the barrier is higher with these other nucleoside analogs. There have been several clinical trials examining emtricitabine as part of a combination antiretroviral regimen. However, given that emtricitabine is a derivative of the widely used compound lamivudine, it is surprising that there have not been any published trials directly comparing these two drugs. There is one unpublished trial, the FTC-302 trial, conducted by industry in South Africa – the key findings are described below.
Related articles And Qustion
See also
Lastest Price from Emtricitabine manufacturers
US $0.00/kg2025-11-27
- CAS:
- 143491-57-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $0.00-0.00/kg2025-04-21
- CAS:
- 143491-57-0
- Min. Order:
- 1kg
- Purity:
- 98%-102%
- Supply Ability:
- 500KGS