Effect of Ropinirole in the Treatment of Parkinson Disease
Levodopa is the cornerstone of symptomatic therapy for Parkinson disease (PD). Long-term use of levodopa, however, is complicated by the potential development of dyskinesia and fluctuations in efficacy. The use of alternate therapies, such as dopamine agonists, in the initial treatment of patients with early PD has been investigated. There is an interest in the use of early dopaminergic agonist monotherapy to delay the initiation of levodopa therapy because this may be associated with fewer motor complications and improved long-term outcome.
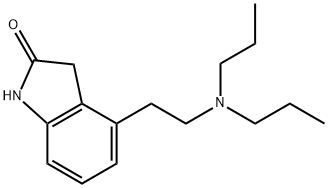
Ropinirole hydrochloride, a nonergoline dopamine (D2) receptor agonist, has been shown to be effective in controlling motor symptoms in patients with early PD and is generally well tolerated.

Experiment
A total of 184 of 241 patients successfully completed the initial 6-month study: 79 of 116 patients in the ropinirole-treated group and 105 of 125 patients in the placebo-treated group. Thirty-seven ropinirole-treated patients (31.9%) withdrew from the initial study compared with placebo-treated patients (20 [16.0%] of 125). The leading cause of withdrawal in both treatment groups was attributable to adverse experiences (27 [23.3%] of 116 ropinirole-treated patients and 13 [10.4%] of 125 in the placebo-treated group). Of 184 patients who completed the initial 6-month study, 147 elected to enter the 6-month extension study (70 in the ropinirole-treated group and 77 in the placebo-treated group). Although a greater percentage of patients in the placebo-treated group (84%) completed the initial 6-month study compared with the ropinirole-treated group (68%), a greater percentage of ropinirole-treated patients (70 [88.6%] of 79) elected to enter the 6-month extension study compared with the placebo-treated patients (77 [73.3%] of 105) . A similar percentage of patients completing 12 months of therapy was observed in both treatment groups (65 [56%] of 116 in the ropinirole-treated group and 69 [55%] of 125 in the placebo-treated group).
Adverse experiences led to the withdrawal of 7 patients (3 patients [4.3%] in the ropinirole-treated group and 4 patients [5.2%] in the placebo-treated group) from the 6-month extension study. None of these patients had received levodopa therapy. No other reason (ie, lack of efficacy) led to the premature discontinuation of more than 2 patients in either treatment group.
The demographic profile and baseline PD characteristics of the 6-month extension study population were similar to those of the initial 6-month study population. Thirty-six (52%) of 70 patients in the ropinirole-treated group and 40 (52.0%) of 77 patients in the placebo-treated group received selegiline concomitantly. By week 6 in the initial study, 104 patients in the ropinirole-treated group had a mean±SD total daily dose of 5.3 ±1.1 mg and 115 patients in the placebo-treated group had a mean (±SD) total daily dose of 5.4 ±1.1 mg. Patients continuing in the extension study remained taking similar doses of study medication compared with the initial 6-month study. The mean (±SD) total daily dose of study medication at end point (48 weeks) was 17.9 (±7.0) mg in the ropinirole-treated group (15.7±8.3 mg at 6 months) and 22.2 (±4.7) mg in the placebo-treated group (19.7±7.1 mg at 6 months).
Conclusion
12-month treatment with ropinirole continued to provide effective symptomatic control in patients with early PD and was generally well tolerated. Ropinirole-treated patients continued to do well over the 6-month extension study without initiation of levodopa therapy. Overall, the number of patients who successfully completed the 12-month study and did not receive additional symptomatic therapy with levodopa was much greater for the ropinirole-treated group compared with the placebo-treated group. These results extend the findings of the initial 6-month study, and support the use of ropinirole as an effective initial option for the treatment of patients with early PD.
Reference
[1] Sethi KD, O'Brien CF, Hammerstad JP, et al. Ropinirole for the Treatment of Early Parkinson Disease: A 12-Month Experience. Arch Neurol. 1998;55(9):1211–1216.
Lastest Price from Ropinirole manufacturers

US $0.00-0.00/KG2025-05-21
- CAS:
- 91374-21-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1KG 100KG 1MT

US $90.00-80.00/kg2024-08-12
- CAS:
- 91374-21-9
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 2000kg