Donepezil Hydrochloride: Structure-Activity Relationships and Pharmacological Characteristics
Donepezil hydrochloride is an acetylcholinesterase inhibitor most commonly used for the treatment of Alzheimer disease.

Structure-Activity Relationships
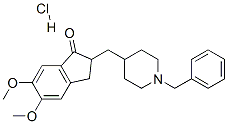
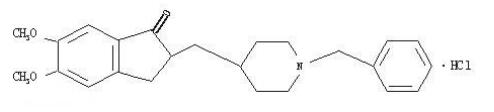
The hydrochloride salt of a piperidine derivative with neurocognitive-enhancing activity. Donepezil Hydrochloride reversibly inhibits acetylcholinesterase, thereby blocking the hydrolysis of the neurotransmitter acetylcholine and, consequently, increasing its activity. This agent may improve neurocognitive function in Alzheimer's disease, reduce sedation associated with opioid treatment of cancer pain, and improve neurocognitive function in patients who have received radiation therapy for primary brain tumors or brain metastases.
Pharmacological Characteristics
Donepezil hydrochloride, a piperidine derivative, is a centrally acting, rapid, reversible acetylcholinesterase inhibitor. Acetylcholinesterase is an enzyme that breaks down acetylcholine after its release from the presynapse. Donepezil binds reversibly to acetylcholinesterase, preventing acetylcholine hydrolysis, thereby increasing the availability of acetylcholine at the synapses and enhancing cholinergic transmission. In vitro data suggest that the anticholinesterase activity of donepezil is relatively specific for acetylcholinesterase in the brain. Donepezil is structurally unrelated to other anticholinesterase agents such as tacrine and physostigmine.1In addition to its cholinergic effects, some noncholinergic mechanisms have been proposed for donepezil.
Uses
Donepezil hydrochloride, a piperidine derivative, is a centrally acting, rapid, and reversible acetylcholinesterase inhibitor primarily utilized for treating Alzheimer disease.
Reference
1. Seltzer B. Donepezil: a review. Expert Opin Drug Metab Toxicol. 2005 Oct;1(3):527-36.
Related articles And Qustion
See also
Lastest Price from Donepezil Hydrochloride manufacturers

US $0.00-0.00/kg2025-07-23
- CAS:
- 120011-70-3
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 1500kg/month

US $0.00-0.00/kg2025-04-21
- CAS:
- 120011-70-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 850kg