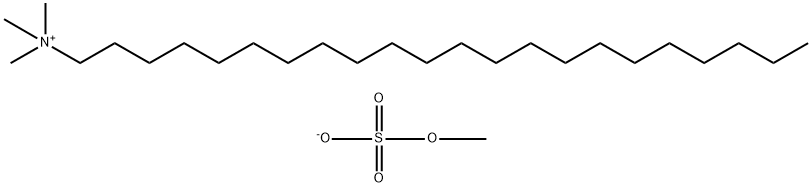
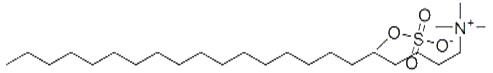
Docosyltrimethylammonium Methyl Sulphate: Cosmetic Applications, Safety, and Environmental Concerns
Introduction
Docosyltrimethylammonium methyl sulphate plays a variety of roles in cosmetic formulations including as an emulsifier and surfactant. It is noted in research as being “skin friendly” because it does not impair intercellular lipids. It is especially popular in hair care formulations geared for curly textures where it serves as a conditioning agent to keep hair static-free, moisturized, and less prone to frizzing. Docosyltrimethylammonium methyl sulphate is also noted for its ability to enhance the delivery of actives to skin. Docosyltrimethylammonium methyl sulphate is categorized as quaternary ammonium salt (water-soluble positively charged ions). As a raw material, it takes the form of pale-yellow pellets. This ingredient is noted for compatible stability over a wide pH range. In 2012, the Cosmetic Ingredient Review Expert Panel deemed docosyltrimethylammonium methyl sulphate safe as used in personal care products. Their report looked at products containing up to 10% concentration, though typically usage levels are much lower (around 0.5%-2%). [1] [2]
Applications as Hair Conditioning Agents
One of the most complex problems in hair care formulations is the duality of the surfactants used. In this regard, such surfactants must be cationic so as to interact with the negatively charged cuticle surface of hair. However, these interdependencies typically lead to non-ideal values for the required hydrophilic–lipophilic balance (HLB) in the oil phase. The cationic surfactants docosyltrimethylammonium methyl sulphate were evaluated for the potential use in hair conditioners. The surfactants were evaluated for their capability to decrease the surface tension in an aqueous solution through contact angle measurements between the oily phase and the aqueous phase. The required HLB of the oil phase was also determined. The emulsification process was developed using standard preparation methods. For three months, the prototypes with high viscosity were packed in containers and stored in a stability chamber at accelerated conditions (40 ± 2 ◦C and 75 ± 5% RH). During this time, the size, polydispersity, zeta potential, viscosity, rheological profile, and creaming index were all evaluated monthly. The results showed a slight change in the physical stability of the prototypes, where the droplet size increased moderately, however, did little to destabilize the formulations. It was found that the required HLB for the oil phase used was between 12 and 13, but the cationic surfactant blends that most approached this value were incapable of reaching the viscosity values required for these types of products. Therefore, it concludes that the best surfactant blend cannot be explained by the HLB theory because of its cationic nature. This suggests that the mixtures of docosyltrimethylammonium methyl sulphate surfactants used could be useful for technological developments in hair conditioning products. [3]
Preparation
Docosyltrimethylammonium methyl sulphate (behentrimonium methosulfate) can be prepared by the addition of N, N-dimethyldocosan-1-amine to dimethyl sulfate. [4]

Environmental Pollution Risk
Docosyltrimethylammonium methyl sulphate belongs to alkyltrimethylammonium compounds. The distribution of alkyltrimethylammonium compounds (ATMAC), cationic surfactants used in a wide variety of applications, has been determined in sediments from Jamaica Bay (NY). Total concentrations in surficial sediments collected between 1998 and 2008 ranged from 361 to 6750 ng/g. The highest values were found in samples from a deeper basin directly affected by treated wastewater discharges. Docosyltrimethylammonium methyl sulphate, a mixture dominated by a homologue having 22 carbon atoms in its alkyl chain (ATMAC 22), was identified for the first time using time-of-flight mass spectrometry and accounted for approximately 80% of the total alkyltrimethylammonium compounds in recent sediment samples. Analyses of a dated sediment core and subsequent surface grab samples revealed an exponential increase in concentration over the last three decades with a doubling time of 3.9 years. Similar temporal trends were seen in surface samples from other sites in Jamaica Bay and Newton Creek (NY), another site greatly influenced by wastewater discharges. This dramatic increase in Docosyltrimethylammonium methyl sulphate reflects greater use of behentrimonium and likely replacement of other products containing other alkyltrimethylammonium compounds homologues in personal care products. Further monitoring is recommended to assess the environmental risk and fate of this persistent emerging contaminant. [5]
References:
[1] Journal of Cosmetic Dermatology , January 2018, pages 140-144.
[2] Journal of Allergy & Therapy, 2014, pages 1-8.
[3] Agredo, P., Rave, M. C., Echeverri, J. D., Romero, D., & Salamanca, C. H. (2019). An evaluation of the physicochemical properties of stabilized oil-in-water emulsions using different cationic surfactant blends for potential use in the cosmetic industry. Cosmetics, 6(1), 12.
[4] Becker, L. C., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., Liebler, D., ... & Andersen, F. A. (2012). Safety assessment of trimoniums as used in cosmetics. International journal of toxicology, 31(6_suppl), 296S-341S.
[5] Lara-Martín, P. A., Li, X., Bopp, R. F., & Brownawell, B. J. (2010). Occurrence of alkyltrimethylammonium compounds in urban estuarine sediments: behentrimonium as a new emerging contaminant. Environmental science & technology, 44(19), 7569-7575.
Related articles And Qustion
See also
Lastest Price from docosyltrimethylammonium methyl sulphate manufacturers

US $100.00/kg2025-11-14
- CAS:
- 81646-13-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $25.00-1.00/g2025-11-03
- CAS:
- 81646-13-1
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 600tons