Diphenylmethane: Natural Occurrence, Activity and Preparation Method
General Description
Diphenylmethane is a naturally occurring compound found in potatoes and soybeans. Recent research has identified diphenylmethane derivatives with dual agonistic activity towards PPARα and PPARγ, showing therapeutic potential for conditions like insulin resistance. These derivatives exhibit anti-proliferative effects and enhance mitochondrial function through PPARα activation. Diphenylmethane can be synthesized through the Suzuki coupling reaction using phenylboronic acid and benzyl chloride. The synthesis process involves specific reactants, reagents, and a catalytic system, resulting in a 100% yield of diphenylmethane. This method highlights the compound's significance in both natural sources and potential therapeutic applications.

Figure 1. Diphenylmethane
Natural Occurrence
Diphenylmethane, a chemical compound, is reported to naturally occur in certain food sources, according to the VCF (Volatile Compounds in Food) database. Specifically, it has been found in potato (Solanum tuberosum L.) and soybean (Glycine max. L. merr.). The VCF database, curated by Nijssen et al., provides comprehensive information on volatile compounds present in natural and processed food products. It serves as a valuable resource, incorporating data from various sources including FEMA GRAS (Generally Recognized as Safe) and EU-Flavis. These findings highlight the natural presence of diphenylmethane in select food items, indicating its potential role as a constituent in their aroma or flavor profiles. While the exact concentrations and implications of diphenylmethane in these foods may vary, its identification underscores the complexity of food composition and the diversity of naturally occurring compounds within edible plant sources. Further research into the presence and significance of diphenylmethane in foods could contribute to a deeper understanding of food chemistry and may have implications for food safety, flavor development, and nutritional analysis. 1
Activity
The recently discovered diphenylmethane derivatives demonstrate potent dual agonistic activity towards both peroxisome proliferator-activated receptor alpha (PPARα) and gamma (PPARγ), showcasing significant therapeutic promise. Identified through meticulous screening, these diphenylmethane derivatives emerge as partial agonists for both receptors, with compound 1 exhibiting particularly robust efficacy. Notably, both enantiomers of compound 1 exhibit comparable transactivation activity, indicative of a distinctive mode of action. Further investigation reveals that (S)-1 exerts notable anti-proliferative effects, partly attributed to its inhibition of the Wnt/β-catenin signaling pathway often implicated in colorectal cancer. Additionally, these diphenylmethane derivatives demonstrate a significant impact on mitochondrial function, activating the carnitine shuttle system by upregulating key genes like the carnitine/acylcarnitine carrier (CAC) and carnitine-palmitoyl-transferase 1 (CPT1). This activation fosters enhanced mitochondrial biogenesis, crucial for cellular energy metabolism and homeostasis. Crucially, these effects are mediated via PPARα, establishing a positive regulatory feedback loop. In summary, the newly identified diphenylmethane derivatives exhibit dual agonistic activity towards PPARα/γ, offering novel anti-proliferative effects and robust activation of mitochondrial function and biogenesis. These findings underscore their potential as therapeutic agents for conditions such as insulin resistance, where targeting mitochondrial function could prove beneficial. 2
Preparation Method
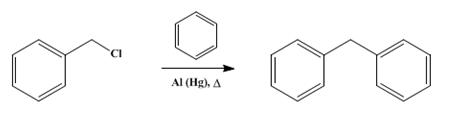
Diphenylmethane, a key organic compound, can be synthesized through the Suzuki coupling reaction. The reactants include phenylboronic acid and benzyl chloride, while the reagents comprise cesium carbonate and tetrabutylammonium bromide. The catalytic system involves (SP-4-2)-Dichloro[N-[(3,5-dimethyl-1H-pyrazol-1-yl-κN2)methyl]-3,5-dimethyl-N-(2,4,6-trimethylphenyl)-1H-pyrazole-1-methanamine-κN2]palladium. To execute the synthesis, a 25 mL vial is charged with benzyl chloride (1.00 mmol), phenylboronic acid (1.20 mmol), Cs2CO3 (0.487 g, 1.50 mmol), TBAB (0.484 g, 1.5 mmol), and diethyleneglycol-di-n-butyl ether (internal standard) (0.218 g, 1.00 mmol). The addition of {N,N(bis(3,5-dimethylpyrazolyl)methyl)-2,4,6-trimethylaniline}PdCl2(2 mol%) is followed by the solvent (DMF-H2O, 9 : 1 v/v, 8 mL). The reaction mixture is then heated at 120 °C for 5 hours. Afterward, an aliquot is filtered, and Diphenylmethane is analyzed using gas chromatography, with diethyleneglycol-di-n-butyl ether serving as an internal standard. This method facilitates the successful production of Diphenylmethane, with a yield of 100%. The process falls under the category of the Suzuki coupling reaction, specifically involving the coupling of aryl compounds with arylboronic acid derivatives. 3
Reference
1. Diphenylmethane. Volatile Compounds in Food. CAS: 101-81-5.
2. Piemontese L, Cerchia C, Laghezza A, et al. New diphenylmethane derivatives as peroxisome proliferator-activated receptor alpha/gamma dual agonists endowed with anti-proliferative effects and mitochondrial activity. Eur J Med Chem. 2017; 127: 379-397.
3. John A, Shaikh MM, Butcher RJ, Ghosh P. Highly efficient palladium precatalysts of homoscorpionate bispyrazolyl ligands for the more challenging Suzuki-Miyaura cross-coupling of aryl chlorides. Dalton Trans. 2010; 9(31): 353-7363.
Related articles And Qustion
Lastest Price from Diphenylmethane manufacturers

US $10.00/KG2025-04-21
- CAS:
- 101-81-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $120.00/kg2025-04-15
- CAS:
- 101-81-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton