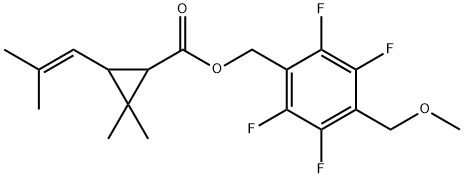
Dimefluthrin: A new pyrethroid insecticide agent
A series of 4-substituted-2,3,5,6-tetrafluorolbenzyl (1R,3R)-chrysanthemates were synthesized and evaluated for their insecticidal activity against Culex pipiens pallens (Coquillett). Among them, the 2,3,5,6-tetrafluoro-4- methoxymethyl analogue (dimefluthrin) exhibited the highest insecticidal activity, and also showed a faster knockdown efficacy towards Cx. pipiens pallens and the southern house mosquito (Culex quinquefasciatus Say) than d-allethrin in a mosquito coil formulation.
Introduction
Natural pyrethrins have been used for a long time as household insecticides, and prallethrin (Shinjo et al., 1989) was commercialized for this purpose as the propynyl analogue of pyrethrin I. d-Allethrin and prallethrin are used extensively throughout the world as the insecticides for controlling mosquitoes. Modification of the alcohol moiety (Ujihara et al., 2004) revealed that 2,3,5,6-tetrafluorobenzyl (1R,3R)-chrysanthemate (1) (Fig. 1) exhibited a faster knockdown activity than d-allethrin. Herein, we report the synthesis of this chrysanthemate and related pyrethroids, and provide some discussion of the structure-activity relationships of these compounds in the insecticidal activities toward two different species of mosquito.
Materials and Methods
1. Synthesis of compounds
NMR spectra were obtained on a BRUKER EX-300 spectrometer (300 MHz) or a JEOL AL400 spectrometer (400 MHz). Chemical shifts (δ) were recorded in ppm, and the coupling constants (J) in Hz. Infrared (IR) spectra were obtained with a HORIBA FT-720 spectrometer. d-Allethrin [2-methyl-4-oxo-3-(2-propenyl)-2-cyclopenten-1-yl2, 2-dimethyl-3- ( 2-methyl-1-propenyl ) cyclopropanecarboxylate] was produced by Sumitomo Chemical.
A typical procedure for the synthesis of the chrysanthemates is given below for the preparation of dimefluthrin (4). [2,3,5,6-Tetrafluoro-4-(methoxymethyl)phenyl]methyl (1R,3R)-2,2-dimethyl-3-(2-methyl-1-propenyl)cyclopropane carboxylate (4). (1R,3R)-2,2-dimethyl-3-(2-methyl-1 -propenyl)cyclopropanecarbonyl chloride (0.90 g, 4.8mmol) was added to a cooled solution(ice-cooling) of [2,3,5,6- -tetrafluoro-4-(methoxymethyl)phenyl]methanol (Mori and Hirose, 2007) (1.0 g, 4.5 mmol) and pyridine (0.42 g, 5.3 mmol) in THF (10 ml), and the resulting mixture was stirred for 8 h at room temperature. The reaction was then poured into 50 ml of ice-cooled water, and the resulting mixture was extracted with EtOAc (2 × 80 ml). The combined extracts were washed with saturated brine and dried over anhydrous Na2SO4 and concentrated under vacuum to give the crude product as a residue, which was purified by column chromatography over SiO2 eluting with a 1:1(v/v) mixture of hexane and EtOAc to give 4 (1.40 g, 84 %) as a colorless oil: 1 H NMR δH (300 MHz, CDCl3): 1.13 (3H, s), 1.26 (3H, s), 1.38 (1H, d, J = 5.2 Hz), 1.69 (6H, brs), 2.10 (1H, dd, J = 5.2, 6.4 Hz, CH-CH-CH=), 3.40 (3H, s), 4.59 (2H, s), 4.87 (1H, d, J = 6.4 Hz), 5.24 (2H, dd, J = 10.5, 11.7 Hz, C-CH2-OC=O); 13C NMR δC (100 MHz, CDCl3): 18.3, 20.2, 21.9, 25.4, 29.1, 33.2, 34.2, 53.3, 58.4, 61.3, 114.9 (1C, dd, J = 18.1, 18.1 Hz), 116.5 (1C, dd, J = 17.1, 17.8 Hz), 120.7, 135.7, 143.9 (2C, ddd, J = 239, 14.3, 5.7 Hz), 146.3 (2C, ddd, J = 250, 14.3, 5.8 Hz), 171.7; 19F NMR δF (376 MHz, CDCl3) :[C6F6 as an internal standard] :18.1 (2F, dd, J = 13.9, 22.2 Hz), 18.8 (2F, dd, J = 13.9, 22.2 Hz); IRνmax cm−1 : 1729, 1488, 1286, 1191, 1149, 1112, 1091, 1056; HRMS m/z [M]+ : Calcd. for C19H22F4O3, 374.15; Found, 374.15. [α] 24D −24 ° (c 0.42, CHCl3). (2,3,5,6-Tetrafluorophenyl)methyl (1R,3R)-2,2-dimethyl-3-(2-methyl-1-propenyl) cyclopropanecarboxylate (1).
2. Preparation of mosquito coils
The test mosquito coils were prepared according to the method described by Yamaguchi et al (1981). Specific amounts of the acetone solutions of d-allethrin and dimefluthrin were pipetted evenly onto the upper surfaces of blank mosquito coils. The blank mosquito coils consisted of pyrethrum marc (50%w/w), Tabu powder (i.e., wood powder behaving as a thickening agent, 30 % w/w), dyestuff (<1%w/w), mildewcide (<1%w/w) and wood flour (balance).
3. Bioassay
3.1. Lethal doses
The lethal doses of the compounds against mosquitoes were tested by the topical application to female adults (3–5 days old) of Cx. pipiens pallens according to the method described by Yamaguchi et al (1981). Each of the test compounds were diluted in acetone and topically applied to the dorsal prothorax of the test insects. Ten insects were treated with each substance/dose and the tests were conducted in triplicate. The LD50 values (i.e., doses required to kill 50% of the test insects) were calculated using the probit method (Bliss, 1938).
3.2. Knock-down efficacy
A fixed amount (0.5 g) of the test coil was fitted to a coil holder and placed at the center of a medium-size glass chamber (ca. 5.8 m3 ). The coil was then ignited and allowed to burn. Immediately after the coil burnt out, 20 female adults (3–5 days old) of Cx. pipiens pallens or Cx. quinquefasciatus were released into the chamber. The number of knocked-down mosquitoes was counted at designated time-points for a period of 30 minutes. The experiments for the dimefluthrin and d-allethrin plots were conducted in duplicate, and the KT50 values (50 % Knock-down Time) were calculated using the probit method (Bliss, 1938).
You may like
Lastest Price from Dimefluthrin manufacturers

US $0.00/kg2025-03-07
- CAS:
- 271241-14-6
- Min. Order:
- 25kg
- Purity:
- 95%
- Supply Ability:
- 50MT/Month

US $19.10/KG2024-10-11
- CAS:
- 271241-14-6
- Min. Order:
- 1KG
- Purity:
- 96%
- Supply Ability:
- 10 ton