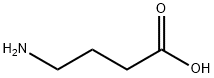
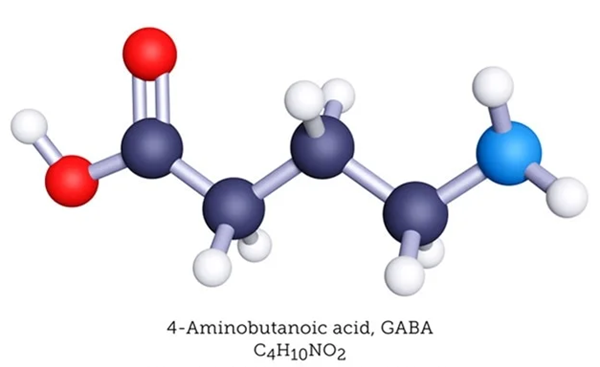
Different synthetic methods of 4-aminobutyric acid
Introduce
4-Aminobutyric acid (GABA) is a compound with the chemical formulaC4H9NO2, is an amino acid, which widely exists in vertebrates, plants and microorganisms. [1] 4-Aminobutyric acid is an important inhibitory neurotransmitter in the central nervous system, which has good water solubility and thermal stability. It has been confirmed that GABA, as a small molecular weight non protein amino acid, has edible safety and can be used in the production of beverages and other foods. Studies have shown that a certain amount of GABA can improve the body's sleep quality and reduce blood pressure.

Picture 1 4-Aminobutyric acid health products
Performance
4-Aminobutyric acid alias 4-aminobutyric acid( 4-Aminobutyric acid (GABA) is an amino acid with chemical formula: h2nch2ch2cooh; Molecular weight: 103.1. GABA is white crystal powder without optical rotation. [2] Melting point 195-204 ℃ (decomposition), miscible with water, slightly soluble in ethanol and acetone, insoluble in benzene and ether, water will be lost during decomposition to form pyrrolidone.
GABA often exists in the form of zwitterions (negatively charged carboxyl group and positively charged amino group) in solution. Due to the electrostatic interaction between positively and negatively charged groups, GABA can have both gaseous (folded) and solid (Extended) molecular conformations in solution, while the coexistence of multi molecular conformations in solution enables GABA to bind a variety of receptor proteins and play a variety of important physiological functions.
Preparation method
In 1993, a scholar successfully developed GABA by chemical synthesis for the first time. Since then, the relevant research has become increasingly rich. In order to obtain more GABA, researchers began various attempts and achieved many results.
Chemical synthesis
The more important chemical synthesis mainly includes the following: the first is the use of potassium Phthaloyl imine and γ- Chloroprene cyanogen or butyrolactone is used as the raw material of GABA. The final product obtained after violent reaction and hydrolysis is GABA; The second is to use pyrrolidone as the initial raw material, hydrolyze it through calcium hydroxide and ammonium bicarbonate, and finally open its ring to obtain GABA; The third is to use butyric acid and ammonia as raw materials of GABA γ GABA was obtained by light reaction under X-ray conditions; The fourth method is to synthesize GABA with propylamine and formic acid by glow discharge; The fifth is to use methyl bromoacetate and ethylene as raw materials to prepare GABA. Methyl 4-bromobutyrate is obtained through polymerization. Finally, the product after ammonolysis and hydrolysis is GABA. The chemical synthesis methods of GABA have the disadvantages of difficult reaction control and high cost.
Plant enrichment method
Plant enrichment method is a newly developed synthetic extraction and purification technology. It uses plants with high GABA content for separation and extraction, so as to have GABA products with both cheap and high purity. There are two main methods to obtain GABA from plants: one is solvent extraction and purification, and the other is column separation and preparation.
Solvent extraction uses water or alcohol as the extraction agent of GABA. According to the principle of different solubility and partition coefficient of plants in water or alcohol, GABA is put into water or alcohol, and after repeated filtration and purification, most of GABA in plants can be extracted. [2]
Column separation preparation method, also known as column chromatography, is a kind of elution separation and other subsequent operations based on the principle that the components of different mixtures have different partition coefficients in solid-liquid two phases. Its large classification should belong to chromatography. Resin, silica gel or activated carbon are generally used as filling materials for chromatographic columns. [2]
Microbial fermentation
Microbial fermentation is to select strains with excellent varieties, stability, non-toxic and harmless, and use these strains to prepare and produce GABA in the process of growth and reproduction. Although this method has strict requirements on the environment and equipment, the GABA produced by this method can be used as a natural food additive. Microbial fermentation production is one of the earliest and most extensive production methods in the food industry. The earliest microorganism used is Escherichia coli. GABA can be produced by using its decarboxylase. However, it has been unable to be directly used in the production of drugs or food due to some potential safety hazards. [2]
With the development of science and technology, green food has attracted more and more attention. Later, researchers found that lactic acid bacteria, yeast, Aspergillus and other microorganisms can be used to replace Escherichia coli and catalyze the production of GABA. Moreover, under the condition of low cost, it also has the advantages of high output and good safety. This method has gradually developed to industrialized production.
Application
The content of GABA in plant tissue is very low, usually 0.3 ~ 32.5 μ Mol / g. It has been reported that the enrichment of GABA in plants is related to the stress response experienced by plants. When subjected to hypoxia, heat shock, cold shock, mechanical damage, salt stress and other stress pressures, it will lead to the rapid accumulation of GABA. After some stress treatment of plant food raw materials, or through microbial fermentation, the content of GABA in plant food raw materials is increased. Processing this raw material into GABA rich functional products has become a research hotspot. As a new functional factor, GABA has been widely used in the field of food industry. Foods developed from germinated brown rice, soybeans and broad beans rich in GABA have been put on the market.
Reference
1 Baxter C F. The nature of γ-aminobutyric acid[M]//Metabolic Reactions in the Nervous System. Springer, Boston, MA, 1970: 289-353.
2 Shelp B J, Bown A W, McLean M D. Metabolism and functions of gamma-aminobutyric acid[J]. Trends in plant science, 1999, 4(11): 446-452.
Related articles And Qustion
Lastest Price from 4-Aminobutyric acid manufacturers

US $0.00/kg2025-09-22
- CAS:
- 56-12-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $0.00/kg2025-08-27
- CAS:
- 56-12-2
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 20tons