Diethyl Malonate: Versatile Building Block in Organic Synthesis
Introduction
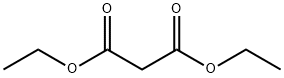
Diethyl malonate, a colorless, fragrant ester of malonic acid, is increasingly significant in the realm of organic chemistry. Known chemically as diethyl propanedioate, this compound is not only pivotal in synthesis but also a cornerstone in the production of numerous pharmaceuticals, flavors, and fragrances. Its versatile nature and chemical reactivity make it an essential ingredient in the toolkit of chemists and researchers. Often utilized for its efficacy in nucleophilic substitution reactions, diethyl malonate serves as a precursor to a wide range of synthetic derivatives. Its unique properties also enable the exploration of new chemical pathways, contributing to innovations in drug development and the creation of novel aromatic compounds. This broad utility underscores its importance and growing prominence in scientific and industrial applications.

Figure 1 Characteristics of Diethyl malonate
Synthesis of Diethyl Malonate
The synthesis of diethyl malonate typically involves the esterification of malonic acid with ethanol. This reaction is catalyzed by strong acids such as sulfuric or hydrochloric acid, facilitating the formation of diethyl malonate through a dehydration mechanism. An alternative method includes the transesterification of dimethyl malonate with ethanol, a process often preferred for its mild reaction conditions and ease of implementation in industrial settings.
Main Components
Diethyl malonate is primarily composed of carbon, hydrogen, and oxygen, reflecting its ester classification. Its molecular structure features two ethoxy groups (OC2H5) linked to a central carbon atom of malonic acid. This ester's reactivity is significantly influenced by the presence of the active methylene group, which is highly reactive due to its adjacent electron-withdrawing ester groups, rendering the hydrogen atoms on the methylene group particularly acidic. This enhanced acidity makes it highly susceptible to deprotonation, forming a resonance-stabilized enolate ion. This critical feature is instrumental in many organic reactions, such as Michael additions and aldol condensations, enabling the formation of more complex molecular structures.
Applications of Diethyl Malonate
The applications of diethyl malonate are diverse and impactful within the chemical synthesis industry. Its primary use is in the Malonic ester synthesis, where it serves as an active methylene compound. This property allows it to undergo alkylation and acylation, facilitating the synthesis of various carboxylic acids and their derivatives. Beyond organic synthesis, diethyl malonate is instrumental in the manufacture of barbiturates, vitamins, and flavoring agents. It also finds applications in the perfume industry, where its ability to contribute subtle, apple-like nuances to fragrance compositions is highly valued. Additionally, diethyl malonate is utilized in the production of synthetic resins and as a starting material in the preparation of several pharmaceutical intermediates. Its role extends into agricultural chemistry as well, where it is used to synthesize pesticides and herbicides that require the unique properties of this versatile ester. The broad scope of its applications highlights the integral role diethyl malonate plays across multiple sectors of the chemical industry.
Storage of Diethyl Malonate
Storing diethyl malonate requires careful consideration to maintain its chemical integrity and prevent hazardous situations. It should be stored in a cool, well-ventilated area away from direct sunlight and sources of heat or ignition. Containers of diethyl malonate need to be tightly sealed to avoid moisture ingress and contamination. Since diethyl malonate is a flammable liquid, it is imperative to adhere to all relevant safety guidelines regarding flammable materials, including proper grounding and bonding of storage containers.
Conclusion
Diethyl malonate's role in organic chemistry extends far beyond its simple molecular composition. As a versatile reagent, it facilitates myriad synthetic pathways, underscoring its importance in the pharmaceuticals, perfumery, and flavor industries. Its effective storage and handling further ensure that diethyl malonate remains a staple in chemical labs and industrial applications worldwide. Understanding its synthesis, applications, and storage is essential for professionals engaged in the wide-ranging fields of chemistry and materials science, highlighting the compound's indispensable nature in modern chemical endeavors.
![Article illustration]() References
References
[1]Hennessy, Edward J., and Stephen L. Buchwald. "A general and mild copper-catalyzed arylation of diethyl malonate."Organic Letters4.2 (2002): 269-272.
[2]Rathke, Michael W., and Patrick J. Cowan. "Procedures for the acylation of diethyl malonate and ethyl acetoacetate with acid chlorides using tertiary amine bases and magnesium chloride."The Journal of organic chemistry50.15 (1985): 2622-2624.
Related articles And Qustion
See also
Lastest Price from Diethyl malonate manufacturers

US $0.00/KG2025-07-09
- CAS:
- 105-53-3
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/g2025-04-21
- CAS:
- 105-53-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg