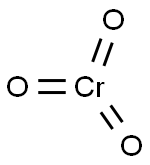
Chromium trioxide - Hazard and Toxicity
Chromium trioxide is not combustible but is a strong oxidizing agent and can accelerate the burning rate of combustible materials. Contact with easily oxidized organic or other combustible materials (including paper and oil) may result in ignition, violent combustion, or explosion. The use of dry chemical, carbon dioxide, Halon, or water spray extinguishers is recommended for fires involving chromium (VI) compounds. Chromium trioxide is used in plating and metal treatment, as a corrosion inhibitor; and as an oxidant; in aluminum anodizing, dye; ink, and paint manufacturing, tanning, engraving; and photography.

Reactivity and Incompatibility
Chromium trioxide and certain other chromium(VI) compounds are useful as strong oxidizing agents in the laboratory, but appropriate precautionary measures should be taken when conducting these reactions. Chromium trioxide has been reported to react violently with a variety of substances, including readily oxidized organic compounds such as acetone, acetaldehyde, methanol, ethanol, diethyl ether, ethyl acetate, acetic acid, and DMF, and violent reactions may also occur on reaction with alkali metals, gaseous ammonia, phosphorus, and selenium.
Mechanism of Chromium Toxicity
Since Cr(III) is poorly absorbed by any route, the toxicity of chromium is mainly attributable to the Cr(VI) form. It can be absorbed by the lung and gastrointestinal tract, and even to a certain extent by intact skin.
The reduction of Cr(VI) is considered to serve as a detoxification process when it occurs at a distance from the target site for toxic or genotoxic effect while reduction of Cr(VI) may serve to activate chromium toxicity if it takes place in or near the cell nucleus of target organs [Dayan and Paine 2001]. If Cr(VI) is reduced to Cr(III) extracellularly, this form of the metal is not readily transported into cells and so toxicity is not observed. The balance that exists between extracellular Cr(VI) and intracellular Cr(III) is what ultimately dictates the amounts and rates at which Cr(VI) can enter cells and impart its toxic effects [Cohen, Kargacin et al. 1993].
Cr(VI) enters many types of cells and under physiological conditions can be reduced by hydrogen peroxide (H2O2), glutathione (GSH) reductase, ascorbic acid, and GSH to produce reactive intermediates, including Cr(V), Cr(IV), thiylradicals, hydroxyl radicals, and ultimately, Cr(III). Any of these species could attack DNA, proteins, and membrane lipids, thereby disrupting cellular integrity and functions [De Mattia, Bravi et al. 2004].
Respiratory Effects
Occupational exposures often include mixed exposure to both Cr(III) and Cr(VI) [EPA 1998].
Human occupational experience clearly indicates that, when inhaled, chromium compounds are respiratory tract irritants, resulting in airway irritation, airway obstruction, and lung, nasal, or sinus cancer. Dose, exposure duration, and the specific compound involved can determine chromium's adverse health effects.
Pulmonary irritant effects following inhalation of chromium dust can include
asthma,
chronic bronchitis,
chronic irritation,
chronic pharyngitis,
chronic rhinitis,
congestion and hyperemia,
polyps of the upper respiratory tract,
tracheobronchitis, and
ulceration of the nasal mucosa with possible septal perforation [Lindberg and Hedenstierna 1983; Dayan and Paine 2001].
Radiographic analysis from several reports revealed enlargement of the hilar region and lymph nodes [PHS 1953; Sluis-Cremer and du Toit 1968]. Consistent associations have been found between employment in the chromium industries and significant risk for respiratory cancer (see Carcinogenic Effects).
A delayed anaphylactoid reaction was reported in a male worker occupationally exposed to chromium vapors from Cr(VI) trioxide baths and chromium fumes from stainless steel welding. A subsequent inhalation challenge with sodium chromate resulted in a reaction including late-onset urticaria, angioedema, and bronchospasm accompanied by tripling of plasma histamine levels [Moller, Brooks et al. 1986].
Many cases of nasal mucosa injury (inflamed mucosa, ulcerated septum, and perforated septum) have been reported in workers exposed to Cr(VI) in chrome-plating plants and tanneries [ATSDR 2000]. A 1983 study of 43 chrome-plating plants in Sweden, where workers were exposed almost exclusively to Cr(VI) acid, revealed that all workers with nasal mucosa ulceration or perforation were periodically exposed to at least 20 micrograms per cubic meter (µg/m³) when working near the plating baths (The newest U.S. permissible exposure level in the workplace for chromates and chromic acid is 5 µg/m³ as a ceiling). The period of exposure for workers experiencing nasal mucosal ulceration varied from 5 months to 10 years [Lindberg and Hedenstierna 1983]. A recent epidemiological study of U.S. workers found that the median time from date first employed to date of first diagnosis of nasal ulceration was less than a month; the median Cr(VI) concentration was similar to concentrations reported in the Swedish study [Gibb, Lees et al. 2000].
Occupational exposure to Cr(III) has also been associated with respiratory effects. One man developed coughing, wheezing, and decreased forced volume after an inhalation exposure to a sample of Cr(III) sulfate [Novey, Habib et al. 1983]. In an industrial hygiene survey of 60 ferrochromium workers exposed to Cr(III) and Cr(VI) (0.02-0.19 mg total chromium/m³) conducted in 1975, appreciably higher incidences of subjective symptoms of coughing, wheezing, and dyspnea were reported compared with controls. However, due to the tobacco smoking that cannot be excluded as a confounding factor, the increase in subjective respiratory symptoms and decreased pulmonary function parameters cannot be unequivocally be attributed to chromium exposure [Langard S 1980].
The respiratory system in animals is also a primary target for inhalation exposure to chromium. Histological examination of the lung tissue revealed alterations representing mild nonspecific irritation after exposure to 0.9 or 25 mg Cr(III) trichloride for 30 min [Henderson, Rebar et al. 1979].
An extensive epidemiological survey was conducted of housewives who lived in an area of Tokyo, Japan, in which contamination from chromium slag at a construction site was discovered in 1973. The exposed population reported a higher incidence of subjective complaints of nasal irritation than the control population in the early years of the study, buy in later years the difference between the two groups became progressively less [ATSDR 2000].

Storage and Handling
Because of their carcinogenicity, chromium(VI) compounds should be handled using the ''basic prudent practices" of Chapter 5.C, supplemented by the additional precautions for work with compounds of high toxicity (Chapter 5.D). In particular, chromium trioxide should be handled in a fume hood to avoid the inhalation of dust, and impermeable gloves should be worn at all times to prevent skin contact. The practice of using chromate solutions to clean glassware should be avoided. Chromium trioxide should be stored in areas separated from readily oxidized materials.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If chromium trioxide or other chromium compounds are ingested, give the person large amounts of water or milk and obtain medical attention immediately. If dust or aerosols of these compounds are inhaled, move the person to fresh air and seek medical attention at once.
In the event of a spill, remove all combustibles from the area, sweep up the chromium compounds, place in an appropriate container, and dispose of properly. In the event solutions containing chromium compounds are spilled, neutralize (if possible) with aqueous base, soak up with a spill pillow or appropriate noncombustible absorbent material, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large spill of powder, particularly in a confined area.
Disposal
Excess chromium compounds and waste material containing these substances should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
Hematological Effects
Cases of hematological effects have been reported in humans after the ingestion of lethal or sublethal doses of Cr(VI) compounds. In a case of an 18-year-old woman who ingested a few grams of potassium dichromate, decreased hemoglobin content and hematocrit, and increased total white blood cell counts, reticulocyte counts, and plasma hemoglobin were found 4 days after ingestion. These effects were indicative of intravascular hemolysis [Sharma, Singhal et al. 1978].
Laboratory analysis of a 35-year-old woman, who died 12 hours after ingesting 50 ml of pure chromic acid [25 g Cr(VI)], revealed anemia (hemoglobin 56 g/L, hematocrit 17 percent) and thrombocytopenia [Loubieres, de Lassence et al. 1999].
Related articles And Qustion
See also
Lastest Price from Chromium(VI) oxide manufacturers

US $9.90/KG2025-04-21
- CAS:
- 1333-82-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 5tons

US $0.00/KG2025-03-05
- CAS:
- 1333-82-0
- Min. Order:
- 1KG
- Purity:
- 99
- Supply Ability:
- 200 MT