Chlorphenesin: Scientific exploration of multipurpose chemicals
Introduction
Chlorphenesin, as a multifunctional chemical, is widely used in many fields such as medicine, cosmetics, and pesticides. Its unique chemical structure and biological activity make it play an important role in these fields. This paper aims to introduce its basic properties, application fields, preparation methods, safety assessment, and safety precautions, in order to provide scientific reference and guidance for researchers, producers, and consumers in related fields.1
Basic nature
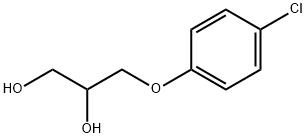
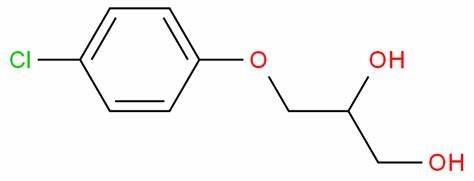
Chlorphenesin, chemically known as 3-(4-chlorophenoxy)-1,2-propylene glycol, is a white to off-white crystalline powder. Its molecular form. C₉HClO₃. Its molecular weight is 202.6. Its melting point is 77-79°C and its boiling point is 369.5°C. It is easily soluble in organic solvents such as ethanol and ether and slightly soluble in water. Its chemical structure is stable, but oxidation reactions may occur under certain conditions.2
Application field
Medical field
In the field of medicine, chlorphenesin is mainly used as an immunosuppressant and an antifungal drug. It can effectively inhibit IGE-mediated histamine release, thereby alleviating allergic reactions. In addition, it also has significant effects on diseases such as fungi, bacteria, vaginal mold, and trichomonas, and is widely used in the treatment of vaginitis and skin diseases.
Cosmetics
In the field of cosmetics, chlorphenesin as an efficient preservative, is widely used in skin care products, shampoos, body washes, toners, and other water-based and emulsion cosmetics. It can effectively resist gram-positive and gram-negative bacteria, especially against Aspergillus Niger, penicillium pine, and other fungi that have strong bactericidal activity, and at the same time against candida albicans and saccharomyces cerevisiae and other yeasts also have a good inhibitory effect. This makes it an indispensable preservative ingredient in cosmetics, which can effectively extend the service life of cosmetics, avoid bacterial or fungal contamination, and ensure the quality and safety of cosmetics.3
Pesticide field
Chlorphenesin also has a certain insecticidal effect and can be used to prevent mosquito bites, head lice body lice, and other diseases. Its insecticidal mechanism is mainly by interfering with the nervous system of pests so that they lose the ability to move and die. However, the application in the field of pesticides is relatively small, and there is a certain risk of toxicity, so its use needs to strictly comply with the relevant regulations and operating procedures.
Preparation method
The main preparation method of Chlorphenesin ether is chemical synthesis. At present, most of the preparation methods reported at home and abroad use p-chlorophenol and epichlorohydrin or epichloropropanol as raw materials to synthesize chlorophenol by chemical reaction. Among them, the method using epichlorohydrin as raw material is widely used, but the reaction conditions of this method are complicated, and it needs to be carried out in two steps, and it needs to use strong oxidants such as sulfuric acid. The method using propylene oxide as raw material is relatively simple, but the raw material of propylene oxide is not easy to obtain, so the popularization and application of this method are limited.4
In the preparation process, it is necessary to strictly control the reaction conditions, such as temperature, pressure, reaction time, etc., to ensure the purity and quality of the product. At the same time, it is also necessary to post-treat the products after the reaction, such as recrystallization, purification, and other steps, to obtain high-purity products.
Security assessment
Although Chlorphenesin ether has a wide range of applications in many fields, its safety issues cannot be ignored. The following are several aspects of its security assessment:
Acute toxicity
Animal experiments show that the acute toxicity of Chlorphenesin is relatively low. However, at high doses, there may still be some toxic effects on animals, such as weight loss, low hemoglobin phenomenon, and decreased spleen and thymus weight. Therefore, when using, the dosage should be strictly controlled to avoid excessive use.
Skin irritation
Chlorphenesin is irritating to the skin. In animal experiments, skin irritation may be observed when a certain concentration is applied to the skin. Therefore, when using chlorine, care should be taken to avoid contact with sensitive areas such as skin or eyes. At the same time, for people with allergies, it should be used with special caution.5
References:
[1] WILBUR JOHNSON. Safety Assessment of Chlorphenesin as Used in Cosmetics.[J]. International Journal of Toxicology, 2014, 33 2 suppl. DOI:10.1177/1091581814526893.[2] ANA RUBIO. Chromatographic-mass spectrometric analysis of the urinary metabolite profile of chlorphenesin observed after dermal application of chlorphenesin-containing sunscreen[J]. Rapid Communications in Mass Spectrometry, 2021, 35 21. DOI:10.1002/rcm.9183.
[3] WEI HONG. Solubility Determination and Thermodynamic Correlation of Chlorphenesin in 12 Pure Solvents from 288.15 to 328.15 K[J]. Journal of Chemical & Engineering Data, 2020, 66 1: 1-858. DOI:10.1021/acs.jced.0c00900.
[4] SANG-EUN LEE K H J Hyang Yeol Lee. Production of chlorphenesin galactoside by whole cells of β-galactosidase-containing Escherichia coli.[J]. Journal of microbiology and biotechnology, 2013, 23 6. DOI:10.4014/jmb.1211.11009.
[5] JING QUAN . Chemo-enzymatic synthesis and sustained release of optically active polymeric prodrugs of chlorphenesin[J]. Polymer, 2008, 49 16: 3353-3610. DOI:10.1016/j.polymer.2008.06.009.
You may like
Related articles And Qustion
See also
Lastest Price from Chlorphenesin manufacturers

US $0.00-0.00/kg2025-07-23
- CAS:
- 104-29-0
- Min. Order:
- 1kg
- Purity:
- 99%min
- Supply Ability:
- 1000kg

US $6.00/kg2025-04-21
- CAS:
- 104-29-0
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000