Carbamazepine:pharmacokinetics, clinical efficacy, and side effects
General Description
Carbamazepine is a widely used antiepileptic drug that offers clinicians many choices for treating epilepsy and trigeminal neuralgia symptoms. However, carbamazepine can cause several adverse drug reactions, including a decrease in sodium levels leading to hyponatremia, cutaneous adverse drug reactions, and immune thrombocytopenia. While generics offer affordability, they may be less consistent due to their multiple manufacturers, which can result in significant side effects or breakthrough seizures. Doctors should also be aware of risk factors for carbamazepine-induced hyponatremia.

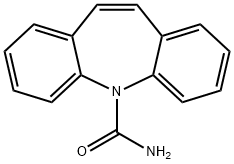
Figure 1. carbamazepine
Pharmacokinetics
Absorption
Carbamazepine is a lipophilic, tricyclic compound slightly soluble in water and is commonly orally administered to children in different formulations. The absolute bioavailability of carbamazepine is estimated to be between 75% and 85%, with a high rate of absorption for suspension and chewable tablets and low rate for extended-release capsules or tablets. Peak plasma concentration varies depending on the formulation, ranging from 1–2 hours for oral suspension to 4–5 hours for conventional tablets. However, absorption in pediatric populations can often be incomplete and erratic due to various factors such as changes in gastric pH, variable activity of enzymes in intestinal metabolism, drug formulations, timing of drug intake in relation to meals, and size of drug doses. 1
Distribution
Carbamazepine is highly bound to plasma proteins, mainly to human serum albumin and alpha1-acid glycoprotein. Its active metabolite, carbamazepine-epoxide, also binds to these proteins. In children, the unbound (free and pharmacologically active) fractions of the parent drug and its metabolite are 25% and 50%, respectively. Both carbamazepine and carbamazepine-epoxide can be measured in plasma, saliva, and cerebrospinal fluid, with saliva being a reliable non-invasive method for estimating their plasma concentrations in children. However, there is great variability in the corresponding plasma levels when measuring concentrations of both compounds in cerebrospinal fluids. 1
Metabolism
Carbamazepine undergoes several metabolic pathways resulting in the identification of 33 metabolites in urine. The major pathway involves the transformation of carbamazepine through oxidation and hydration to produce carbamazepine epoxide, which is conjugated with glucuronide derivative for elimination in urine. This metabolic pathway is primarily catalyzed by CYP enzymes, with CYP3A4 being the most significant. Other pathways include the formation of hydroxyl derivatives by CYP1A2 enzymes and the formation of carbamazepine-acridan. Only a small fraction of carbamazepine is excreted unchanged in urine (1-3% of total dose). 1
Elimination
The clearance of carbamazepine is primarily metabolic rather than renal, with a mean clearance value varying from 0.05 to 0.1 l/h/kg in children. Only a small amount of carbamazepine is excreted unchanged in urine, while its elimination half-life during steady state varies from 4 to 12 hours. Additionally, the elimination half-life of carbamazepine may differ depending on age and body weight, ranging from 6.23 hours in children with epilepsy to 24.5 hours in neonates. 1
Clinical efficacy
Epilepsy
Carbamazepine is an older antiepileptic drug that is widely used and recognized by neurologists and non-neurologists for its spectrum of efficacy, advantages, and limitations. It belongs to a class of AEDs that also includes newer compounds such as oxcarbazepine and eslicarbazepine acetate, as well as various formulations of carbamazepine, including brand name and generic options. While generics offer affordability, they may be less consistent due to their multiple manufacturers, which can result in small differences in pharmacokinetics that lead to significant side effects or breakthrough seizures. Nonetheless, the American Academy of Neurology generally accepts generics as an option for treatment, but automatic substitutions without notifying the neurologist should not be made routinely. Overall, this family of AEDs offers clinicians many choices for treating epilepsy. 2
Tic douloureux
Carbamazepine is considered the first-line medication for the initial medical treatment of trigeminal neuralgia symptoms. It has been shown to increase pain relief compared with placebo. Doctors usually prescribe carbamazepine for trigeminal neuralgia, and it's been shown to be effective in treating the condition. 3
Side effects
Hyponatremia
One of the most common adverse drug reactions associated with Carbamazepine is a decrease in sodium levels leading to hyponatremia, which can occur in up to half of patients with epilepsy taking the medication. Though often assumed to be asymptomatic, it can lead to symptoms ranging from unsteadiness and mild confusion to seizures and coma. Risk factors for carbamazepine-induced hyponatremia include the concomitant use of drugs that cause hyponatremia, menstruation, psychiatric conditions, surgery, psychogenic polydipsia, female gender, and high carbamazepine dosage. 4
Cutaneous adverse drug reaction
Carbamazepine can cause cutaneous adverse drug reactions in which the skin is the main tissue affected. The mechanism of CARBAMAZEPINE-induced cutaneous adverse drug reactions involves either direct cellular damage by the drug or its metabolites or an immune-mediated hypersensitivity process. 4
Thrombocytopenia
Carbamazepine-induced immune thrombocytopenia is categorized as “quinine-type” drug-induced immune thrombocytopenia DITP due to platelet destruction by reticuloendothelial system only in the presence of the drug. 5
Reference
1. Djordjevic N, Jankovic SM, Milovanovic JR. Pharmacokinetics and Pharmacogenetics of Carbamazepine in Children. Eur J Drug Metab Pharmacokinet, 2017, 42(5):729-744.
2. Gierbolini J, Giarratano M, Benbadis SR. Carbamazepine-related antiepileptic drugs for the treatment of epilepsy - a comparative review. Expert Opin Pharmacother, 2016, 17(7):885-888.
3. Tolou-Ghamari Z, Zare M, Habibabadi JM, Najafi MR. ¨ A quick review of carbamazepine pharmacokinetics in epilepsy from 1953 to 2012. J Res Med Sci, 2013, 18:S81–S85.
4. Fricke-Galindo I, LLerena A, Jung-Cook H, López-López M. Carbamazepine adverse drug reactions. Expert Rev Clin Pharmacol, 2018, 11(7):705-718.
5. Chen W, Lv X, Rong R, Wu B. Carbamazepine-induced immune thrombocytopenia confirmed by modified MASPAT test. Transfus Apher Sci, 2021, 60(6):103228.
You may like
Related articles And Qustion
See also
Lastest Price from Carbamazepine manufacturers

US $1.00/KG2025-07-25
- CAS:
- 298-46-4
- Min. Order:
- 1KG
- Purity:
- 98% ~ 102.0%,USP42
- Supply Ability:
- 50kg/month

US $1.00/KG2025-04-21
- CAS:
- 298-46-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt