Bis(pinacolato)diboron: The Invisible Assistant of the Chemical World
Introduction
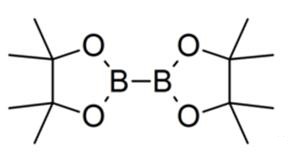
Bis(pinacolato)diboron (CAS number 73183-34-3) is a biochemical reagent widely used in the field of chemistry, especially in organic synthesis. Its molecular formula is C12H24B2O4, its molecular weight is 253.94, and it has unique physical and chemical properties and wide application prospects. This review aims to introduce the properties, synthetic methods, uses, and market development of Bis(pinacolato)diboron, in order to provide a reference for the research and application in related fields.

Physical and chemical properties
Bis(pinacolato)diboron is a white to off-white solid powder with relatively stable physical and chemical properties. Its melting point is 137-140°C, its boiling point is 222.6±7.0°C (at 760 mmHg), and its density is 1.0±0.1 g/cm³. In addition, it has a vapor pressure of 0.2±0.4 mmHg (at 25°C), a flash point of 88.4±18.2°C, and a refractive index of 1.432. These physical and chemical properties require special attention when storing and using bis(pinacolato)diboron, which should be stored in an airtight container and kept in a cool, dry place away from oxidants.1
Synthesis method
Bis(pinacolato)diboron is synthesized by a variety of methods, the most common of which is the reaction of tetramethylamino diboron and pinacol under acidic conditions. This method has the advantages of mild reaction conditions, simple operation, and high yield, so it has been widely used in practical production. In addition, there are other synthesis methods, such as the direct reaction of boric acid and pinacol, the decomposition of borate ester, and other methods to prepare it.2
Application field
As an important biochemical reagent, bis(pinacolato)diboron has been widely used in organic synthesis, material science, and other fields. Specifically, its main applications include the following aspects:3
(1) Organic synthesis
As a boratization reagent in organic synthesis, it can be used for the cis-junction boratization reaction of acetylene and paraffin (platinum-catalyzed), and the boratization reaction of palladium-catalyzed aromatic compounds. These reactions play an important role in organic synthesis and can be used to synthesize a variety of organic compounds with special structures and functions.
(2) Materials science
It also has potential application value in the field of materials science. For example, it can be used as a catalyst ligand for the preparation of nanomaterials with special properties; It can also be used as an additive to improve the physical properties or chemical stability of materials.4
(3) Life science
It also has certain applications in the field of life science. For example, it can be used as a biomaterial or organic compound for life science-related research, providing strong support for biochemical experiments and drug development.
Market development status
With the continuous development of fields such as organic synthesis and materials science, the market demand for bis(pinacolato)diboron is also increasing. Globally, it shows a steady growth trend. In terms of production, China, the United States, Europe, and other regions are its main production places, of which China's output and export volume are in the forefront of the world. In terms of application fields, the field of organic synthesis is its main application field, occupying the majority of the market share. At the same time, with the continuous development of materials science life science, and other fields, its application in these fields will gradually expand.3
Conclusion and Prospect
As an important biochemical reagent, bis(pinacolato)diboron has a wide application prospect in organic synthesis, materials science, and other fields. With the continuous progress of science and technology and the increasing market demand, its research and application will be more in-depth and extensive. In the future, we can expect more breakthroughs and progress in its synthesis methods, application fields, and market development. At the same time, it is also necessary to strengthen its safety and environmental protection research to ensure its safety and sustainability in the production and application process.5
References
[1].Grirrane, A.; álvarez, E.; García, H.; Corma, A., Preparation of Tremorine and Gemini Surfactant Precursors with Cationic Ethynyl‐Bridged Digold Catalysts. Chemistry–A European Journal 2017,23(12), 2792-2801.
[2].Imperio, D.; Pirali, T.; Galli, U.; Pagliai, F.; Cafici, L.; Canonico, P. L.; Sorba, G.; Genazzani, A. A.; Tron, G. C., Replacement of the lactone moiety on podophyllotoxin and steganacin analogues with a 1, 5-disubstituted 1, 2, 3-triazole via ruthenium-catalyzed click chemistry. Bioorganic & medicinal chemistry 2007,15(21), 6748-6757.
[3].Yuan, W.; Ma, S., Ligand Controlled Highly Selective Copper‐Catalyzed Borylcuprations of Allenes with Bis (pinacolato) diboron. Advanced Synthesis & Catalysis 2012,354(10), 1867-1872.
[4].Villarino, L.; López, F.; Castedo, L.; Mascarenas, J. L., Palladium‐Catalyzed Hydroalkynylation of Alkylidenecyclopropanes. Chemistry–A European Journal 2009,15(48), 13308-13312.
[5].Brouwer, F.; Alma, J.; Valkenier, H.; Voortman, T. P.; Hillebrand, J.; Chiechi, R. C.; Hummelen, J. C., Using bis (pinacolato) diboron to improve the quality of regioregular conjugated co-polymers. Journal of Materials Chemistry 2011,21(5), 1582-1592.
Related articles And Qustion
Lastest Price from Bis(pinacolato)diboron manufacturers
US $1.50/g2025-06-25
- CAS:
- 73183-34-3
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 10 Tons

US $0.00/kg2025-04-21
- CAS:
- 73183-34-3
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 50mt