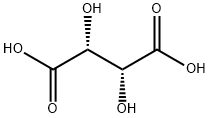
Biosynthesis of L(+)-tartaric acid
Introduction
L(+)-Tartaric acid (TA) is the primary nonfermentable soluble acid in grapes and the principal acid in wine, contributing important aspects to the taste, mouthfeel, and aging potential of the vinified product[1]. Moreover, exogenous TA is used as a flavorant and antioxidant for a range of foods and beverages, including grape juice and wine. Many plants make TA, yet in contrast to the fruit acids malic and citric, pioneering work in the 1950s showed that the metabolic origin of TA lay outside the oxidative metabolism of sugars .

Picture 1 L(+)-tartaric acid powders
TA biosynthesis
Uniquely for a fruit acid, TA biosynthesis begins with L-ascorbic acid (AA, vitamin C) [2]. A key step in TA formation is the cleavage of a six-carbon intermediate between either position C2 C3 or C4 C5, depending on the plant species. The former reaction yields oxalic acid (OxA) and L-threonate, which in plants of the Geraniaceae is then converted to TA in the leaves, whereas the latter ‘‘direct pathway’’ yields TA and a two-carbon compound, possibly glycoaldehyde, and is the pathway proposed to predominate in the Vitaceae. It was recently shown using intact berries of Vitis vinifera that OxA and TA are formed by AA catabolism in these tissues, suggesting that, in the grapevine, both pathways are functional.
The catabolism of AA to TA
In grape berries, TA biosynthesis is limited to the period of development from postanthesis until the onset of ripening or ‘‘veraison’’ . Radioisotope tracer studies reveal that catabolism of AA to TA in the direct pathway proceeds via the conversion of AA to 2-keto L-idonic acid, with successive reduction to L-idonic acid and oxidation to 5-keto D-gluconic acid[3]. In the penultimate step, 5-keto D-gluconic acid is cleaved between carbons to yield the four-carbon L-threo-tetruronate , which is oxidized to form TA. It is proposed that a glycoaldehyde is formed from the remaining two-carbon fragment. Kinetic analyses using 14C-labeled intermediates suggest that oxidation of idonate to 5-keto D-gluconic acid is the rate limiting step in this pathway . Despite having established the nature of chemical intermediates in TA biosynthesis, none of the enzymes responsible for the proposed reactions have been identified. Moreover, attempts to identify the corresponding enzyme activities in crude cell extracts have been unsuccessful. Cleavage of 5-keto D-gluconic acid was proposed to be nonenzymic because of its close kinetic correlation with respect to 14C conversion into TA , although this work was focused more closely on examining 14C flux rather than enzymatic activities. This hypothesis was subsequently refuted when 18O tracer experiments suggested the cleavage mechanism to involve a putative hydrolase.
The formation of TA
More recently, it has been shown that, in Gluconobacter suboxidans, 5-keto D-gluconic acid can be used as the substrate for a dedicated transketolase[4]. These authors speculated that further oxidation of the resulting four-carbon semialdehyde by a succinate semialdehyde dehydrogenase homolog could result in formation of TA. The paucity of specific biochemical and molecular data pertaining to the mechanism and regulation of TA biosynthesis in grapevines is surprising, given the multibillion dollar value of the crop and the close ties between TA and AA metabolism. The biosynthetic pathway for AA has only recently been solved, and a renaissance of interest has occurred with various ways of increasing vitamin C accumulation proposed, such as overexpressing a key enzyme in a different pathway or by manipulating enzymes involved in its recycling. TA biosynthesis represents an unusual fate for AA, and its accumulation to high levels in ripe berries suggests a highly active metabolic pathway that may compete for AA with those redox-associated functions more usually linked with in vivo AA pools[5].
Reference
1. Pasteur, L. (1860) Compt. Rend. 51, 298.
2. Hale, C. R. (1962) Nature 195, 917–918.
3. Stafford, H. A. (1959) Am. J. Bot. 46, 347–352.
4. Loewus, F. A. & Stafford, H. A. (1958) Plant Physiol. 33, 155–156.
5. Saito, K. & Kasai, Z. (1969) Phytochemistry 8, 2177–2182.
Related articles And Qustion
See also
Lastest Price from L(+)-Tartaric acid manufacturers

US $10.00-1.50/kg2025-11-03
- CAS:
- 87-69-4
- Min. Order:
- 1kg
- Purity:
- 99.5%Min
- Supply Ability:
- 700tons

US $1200.00-1100.00/ton2025-08-07
- CAS:
- 87-69-4
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M