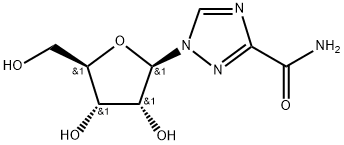
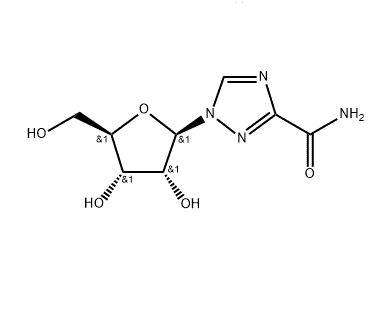
Bioavailability and Toxicity of Ribavirin
Ribavirin (also known as ICN-1229, RTCA, tribavirin), an analog of guanosine, has an unusually wide spectrum of antiviral activity in vitro, perhaps the broadest spectrum against both DNA and RNA viruses of any known antiviral agent other than interferon.
The drug was first described in 1972 and was approved by the Food and Drug Administration (FDA) of the USA in 1986 for aerosol treatment of children with severe respiratory syncytial virus (RSV) infection, although the efficacy of ribavirin for this indication remains controversial. Oral ribavirin was approved by the US FDA in 1998 for treatment of chronic hepatitis C virus (HCV) infection in combination with interferon.
Mechanism of action
The mechanism of action of ribavirin remains controversial, probably because there are multiple mechanisms and these vary with the virus and the cell culture system. Like other nucleoside analogs, ribavirin is a prodrug that enters cells by facilitated diffusion and then rapidly undergoes phosphorylation to the triphosphate form, with mono- and diphosphate derivatives collectively constituting only about 16% of intracellular drug concentrations.
There are five primary mechanisms of action that have been proposed, two indirect and three direct. Indirect mechanisms of action include inhibition of inosine monophosphate dehydrogenase (IMPDH) and enhancement of T-cellmediated immunity favoring a T-helper type 1 cytokine profile. Direct mechanisms of action include inhibition of RNA polymerase, inhibition of RNA capping activity, and an RNA mutagenic effect.
Bioavailability
Oral ribavirin is absorbed in the small intestine via sodium-dependent nucleoside transporters. Oral bioavailability is reported to be between 32.6% and 52%, with evidence of first-pass metabolism. The bioavailability in HIV-infected persons was not significantly different from that in healthy adults. Peak plasma concentrations were observed 1.5 hours after oral administration.
Toxicity
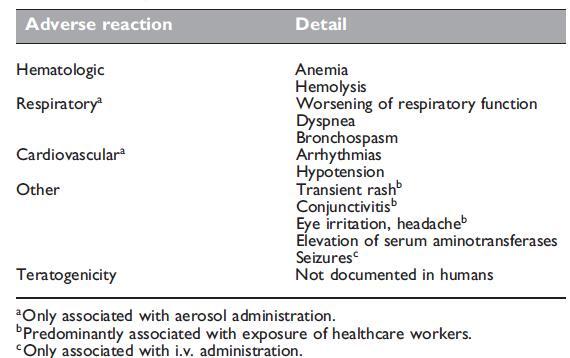
In many clinical trials ribavirin, administered by oral or aerosol routes over a short duration, has been well tolerated and is considered as being a reasonably safe drug for infants, adults including the elderly, and healthcare workers. In comparison, there are far fewer data regarding viramidine. Since viramidine is a prodrug of ribavirin, it has also been shown to inhibit ribavirin phosphorylysis, thereby increasing intracellular concentrations of ribavirin triphosphate, the active moiety. Twenty-eight-day studies in rats demonstrated that viramidine was rapidly converted to ribavirin and had a toxicity profile similar to that of ribavirin; in monkeys, the toxicity profile of viramidine was much better than that of ribavirin.
Like ribavirin, there was no significant impact on cytochrome P450 metabolism. Phase I studies in healthy volunteers evaluating single orally administered viramidine doses of 200, 600, and 1200 mg compared with placebo demonstrated the safety of the compound in humans, with no serious events reported and most adverse events being mild. Another phase I study in HCV-infected subjects at single doses of up to 800 mg twice daily showed viramidine to be safe and well tolerated. Given the greater quantity of data available, this section will focus on ribavirin. A summary of the adverse reactions is shown below in Table.
Teratogenic effects
Ribavirin has teratogenic effects in hamsters and rats if administered during gestation. Developmental malformations involving the limbs, eyes, and brain have been observed after administration of ribavirin to pregnant animals. Ribavirin has also been reported as being mutagenic to bone marrow cells of mice. Tubular atrophy of testes in rats treated with ribavirin has been described. Ribavirin is mutagenic to rat germ cells. Impairment of trophoblast development in female mice treated with ribavirin was associated with an increase in the rate of abortion and retarded rate of embryo development.
You may like
Related articles And Qustion
See also
Lastest Price from Ribavirin manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 36791-04-5
- Min. Order:
- 1KG
- Purity:
- 98.9%-101.5%
- Supply Ability:
- 1000 KGS

US $10.00/KG2025-04-21
- CAS:
- 36791-04-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt