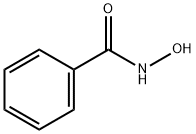
Benzohydroxamic Acid: Synthesis, Applications and Environmental Treatment
Benzhydroxamic acid is used as precursor in the synthesis of novel mono-anionic and di-anionic hydroxamato complexes by reacting with BiPh 3 and Bi (O (t)Bu) 3, which has anti-bacterial activity against helicobacter pylori.

Degradation and Mineralization of Benzohydroxamic Acid
Rare earth element La-doped TiO2 (La/TiO2) was synthesized by the sol-gel method. Benzohydroxamic acid was used as the objective pollutant to investigate the photocatalytic activity of La/TiO2. The physicochemical properties of the prepared materials were characterized by X-ray diffraction, X-ray photoelectron spectroscopy, UV-vis diffuse reflectance spectroscopy, specific surface area and porosity, scanning electron microscopy and transmission electron microscopy. As a result, the doping of La could inhibit the crystal growth of TiO2, increase its specific surface area and expand its response to visible light, thus improving its photocatalytic activity. La/TiO2 with the doping ratio of 0.75% calcined at 500 °C, showing the highest photocatalytic activity to degrade benzohydroxamic acid under the irradiation of 300 W mercury lamp. About 94.1% of benzohydroxamic acid with the original concentration at 30 mg·L−1 was removed after 120 min in a solution of pH 4.4 with an La/TiO2 amount of 0.5 g·L−1. Furthermore, 88.5% of the total organic carbon was eliminated after 120 min irradiation. In addition, after four recycling runs, La/TiO2 still kept high photocatalytic activity on the photodegradation of benzohydroxamic acid. The interfacial charge transfer processes were also hypothesized.[1]
In the Gannan area of China, ion-type rare earth ore is widely distributed. Benzohydroxamic acid is a high-effect chelating collector of oxidized ore, which is widely used in the flotation of lead oxide ore, copper oxide and rare earth ores. It belongs to the aromatic alkyl hydroxamic acid, due to the existence of the π-π conjugated effect, which enhances the stability of the chelate compound by increasing the electron cloud density of atomic oxygen. Benzohydroxamic acid has physiological toxicity due to its benzene ring structure, which increases the COD (Chemical oxygen demand) content of mineral processing wastewater [5]. At the same time, N element content is also increased, which would cause the eutrophication when accumulated in the water. Besides, benzohydroxamic acid is difficult to decompose, and it would remain for a few years or an even longer time once released into the water environment. Eventually, it would bring harm to the water environment and would need to be removed. Therefore, numerous studies have been focused on the photocatalytic activities of rare earth doped TiO2 in recent years, but there are few studies in the treatment of flotation reagents. With the development of mining, mineral processing waste water has become the main source of pollution in the areas of the mine and its surroundings, especially the residual organic flotation reagent, which has high toxicity and high pollution. As far as we know, there is no similar report on the photodegradation of benzohydroxamic acid by the photocatlaytic oxidation technique.
La-doped TiO2 photocatalysts were synthesized by the sol-gel method. XRD diffraction peaks of doped TiO2 were broader and the relative intensity was weaker than pure TiO2. It might improve the thermal stability of the anatase phase of TiO2, suppress particle aggregation, grain growth of TiO2 and increase the specific area of TiO2. The red shift of La/TiO2 in the band-gap transition could reduce gap energy of TiO2 and improve the response strength and threshold value of TiO2 for visible light and extend its absorption side band. La/TiO2 also had a larger specific surface area and a more regular shape in morphology. Furthermore, 0.75% La/TiO2 (500 °C) indicated the highest photocatalytic degradation ability to benzohydroxamic acid: the removal rate and mineralization efficiency of benzohydroxamic acid reached 94.1% and 88.5%, respectively, at the conditions of pH 4.43, 30 mg·L−1 of benzohydroxamic acid, 0.5 g·L −1 of catalyst, and the irradiation of 300 W mercury lamp. The doping of La3+ could reduce the recombination of photoexited electrons and holes, resulting in the improvement of removal efficiency of benzohydroxamic acid by La/TiO2.
Benzohydroxamic acids as potent and selective anti-HCV agents
A diverse collection of 40 derivatives of benzohydroxamic acid (BHAs) of various structural groups were synthesized and tested against hepatitis C virus (HCV) in full-genome replicon assay. Some of these compounds demonstrated an exceptional activity, suppressing viral replication at sub-micromolar concentrations. The compounds were inactive against key viral enzymes NS3, and NS5B in vitro assays, suggesting host cell inhibition target(s). The testing results were consistent with metal coordination by the BHAs hydroxamic group in complex with a target(s). Remarkably, this class of compounds did not suppress poliomyelitis virus (PV) propagation in RD cells indicating a specific antiviral activity of BHAs against HCV. In the present research, we report the synthesis and structure–activity relationship (SAR) studies on HCV inhibition in a full-genome replicon assay for benzohydroxamic acid (BHA) derivatives. Some of the new compounds displayed exceptional antiviral activity and low toxicity for mammalian cells, which makes them promising candidates for drug development.[2]
Reported results demonstrated that benzohydroxamates suppress HCV full-genome replicon propagation in Huh7 cells and allowed us to identify major structural features of Benzohydroxamic acids crucial for their antiviral activity. This class of compounds efficiently (at low micromolar range) suppressed viral proliferation in dose-dependent mode as judged by reduction of luciferase reporter signal and viral RNA content. The antiviral action of Benzohydroxamic acids displayed selectivity and specificity with respect to HCV replication. The suggested inhibitory mechanism was consistent with metal-mediated binding of BHAs to a target through chelation. Our preliminary data point to matrix metalloproteinase-2 and histone deacetylase 6 as possible cellular enzymes responsible for antiviral Benzohydroxamic acids action. The next challenge is further testing of this hypothesis and elucidation of the exact mechanism through which the Benzohydroxamic acid compounds affect HCV proliferation.
Development of effective biotransformation process for benzohydroxamic acid production
Hydroxamic acids (R-CONHOH), known for their ability to form stable chelates with metal ions, find applications as growth factors, food additives, antibiotics, antifungal agents, tumor inhibitors, siderophores, enzyme inhibitors and bioremediation. These compounds are being synthesized by chemical routes which includes N-alkylation of simple o-substituted hydroxylamine with a variety of alkylating agents or by the direct oxidation of lysine and ornithine using dimethyldioxirane. The synthesis of hydroxamic acids by chemical processes is accompanied with by-products; thus, further processing is required to get pure hydroxamic acids and their derivatives. One such important hydroxamic acid which finds application in pharmaceutical industries is benzohydroxamic acid. Many bioactivities have been attributed to BHA including anti-HIV, antimicrobial, antineoplastic agent, antianemic, and potential inhibitor of leukemia and in the treatment urea plasma. Considering the aforementioned properties of Benzohydroxamic acid, it is indeed pertinent to carry out its green synthesis using amidase.[3]
The present study entails the usefulness of thermophilic amidase-producing bacterium in the biotransformation of benzamide to benzohydroxamic acid. A bacterium Bacillus smithii IIIMB2907 was isolated from a soil sample collected from hot springs of Manikaran, Himachal Pradesh, India. The whole cells of the bacterium displayed versatile substrate specificity by exhibiting significant activity with a diverse range of amides. In addition, amidase from thermophilic bacterium was induced by adding Ɛ-caprolactam in the mineral base media. The optimum temperature and pH of acyltransferase activity of amidase enzyme were found to be 50 °C and 7.0, respectively. Interestingly, half-life (t1/2) of this enzyme was 17.37 h at 50 °C. Bench-scale production and purification of Benzohydroxamic acid was carried out at optimized conditions which resulted in the recovery of 64% BHA with a purity of 96%. Owing to this, the reported process in the present study can be considered of immense industrial significance for the production of Benzohydroxamic acid.
References
[1]Luo X, Wang J, Wang C, Zhu S, Li Z, Tang X, Wu M. Degradation and Mineralization of Benzohydroxamic Acid by Synthesized Mesoporous La/TiO₂. Int J Environ Res Public Health. 2016 Oct 10;13(10):997.
[2]Kozlov MV, Kleymenova AA, Romanova LI, Konduktorov KA, Smirnova OA, Prasolov VS, Kochetkov SN. Benzohydroxamic acids as potent and selective anti-HCV agents. Bioorg Med Chem Lett. 2013 Nov 1;23(21):5936-40.
[3]Sharma H, Singh RV, Ganjoo A, Kumar A, Singh R, Babu V. Development of effective biotransformation process for benzohydroxamic acid production using Bacillus smithii IIIMB2907. 3 Biotech. 2022 Feb;12(2):44.
You may like
Lastest Price from Benzohydroxamic acid manufacturers
US $0.00/kg2025-11-07
- CAS:
- 495-18-1
- Min. Order:
- 1kg
- Purity:
- 95%
- Supply Ability:
- According to customer requirements

US $10.00/KG2025-04-21
- CAS:
- 495-18-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt