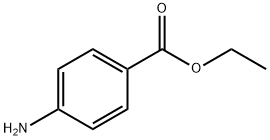
Benzocaine: Mechanism of Action,Uses,Toxicity
General Description
Benzocaine (OrajelR ), 4-aminobenzoic acid ethyl este, is a topical anesthetic agent with analgesic potential commonly employed in oral ulcers. A combination of benzocaine with antipyrine serves to soothe ear pain and eliminate earwax. The most familiar accessible forms of the drugs are oral, topical and otic compositions. Moreover, benzocaine is well-known for its analgesic potency in oral and pharyngeal mucous membranes including mouth cancers, dental and otic discomforts, local anesthesia in surgical procedures, antibacterial, antimicrobial, antifungal, antitumor, redox and relief for mosquito bites.1-2

Figure 1. Properties of Benzocanine
Chemical Nature of Benzocaine
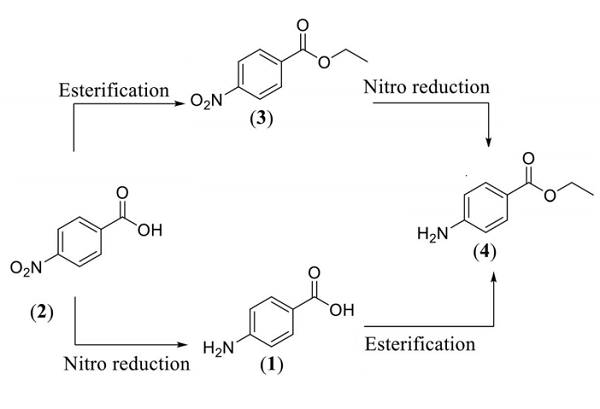
Chemically, benzocaine is the ethyl ester of para aminobenzoic acid (PABA), synthesized by Fischer esterification of para aminobenzoic acid and ethanol or reduction of the nitro group of ethyl para nitrobenzoate to amine. Firstly, the synthesis of the concerned drug was conducted in 1890 by Eduard Ritsert, a German Chemist with "Anasthesin" as the trade name while it was launched in the market in 1902. Initially, the synthesis of benzocaine was accomplished by acid-catalyzed esterification of 4- aminobenzoic acid with ethanol. The molar mass of benzocaine is 165.192 g/mol with 88-90oC melting point and 310°C boiling point. The density value of 1.17g/cm3 has been recorded. The crystals adopt rhombohedral and needle-like shapes when extracted from ether and water, respectively. Solubility studies have shown it to be dissolvable in water (1310 mg/mL at 25oC), more in dilute acids and excessive in organic solvents (1 g/5mL alcohol, 1 g/2 mL chloroform and 1g/4mL ether). Spectral data display IR, UV and 1H NMR peaks to be at 6882, 888 and 1856, respectively.3-4
 Figure 2. Synthesis of Benzocaine
Figure 2. Synthesis of Benzocaine
Mechanism of Action
Benzocaine binds to sodium channels, thereby stabilizing the neuronal membrane. It renders the decrease of permeability to sodium ions; hence, inhibition of the neuronal membrane depolarization is observed along with the blockage of nerve impulse conduction.5
Medical Uses of Benzocaine
As early as 1927, benzocaine-chaulmoogra oil combination was reported to be beneficial in leprosy treatment. The application of benzocaine, ephedrine hydrochloride sol, adrenaline hydrochloride and soft white paraffin revealed a soothing effect on itching in conjunctival and nasal mucous membranes. Benzocaine lozenges were also practiced as anesthetics in surgical procedures and Euphagin, a compound of benzocaine, was considered as a good soporific for sore throat. An emulsion of benzyl benzoate, benzocaine, dichlorodiphenyltrichloroethane (DDT) and tween 80 in water was formulated to be massaged on the area affected with scabies and pediculosis. Benzocaine was applied as a constituent of oil for the cure of pain after rectal operations. Two percent benzocaine in urethane was practiced as a local anesthetic for curing soreness of amputation stumps. Benzocaine in ear drops has been examined to alleviate discomfort . Production of effective tooth pain reliever was brought about by using lidocaine, procaine and benzocaine with lidocaine and procaine present in higher proportion. In dentistry, benzocaine administered solely, diffused numbness in the entire oral cavity, whereas, its concoction with Methocel restricted numbness to the area of application.6
Toxicity
Mild topical applications of benzocaine have not displayed any adverse effects, but over-dosage causes toxicity, thus developing irregular heartbeat and in severe cases, coma and lung problems. Moreover, by using oral sprays with the concentration of benzocaine up to 14-20%, the chances of methemoglobinemia development become prominent in infants and elderlypatients. Benzocaine usage may lead to allergic reactions such as contact dermatitis and anaphylaxis. Besides these, other risk factors associated with benzocaine are genetic disorders and denuding of the skin.7
Pharmacodynamics and Metabolism
Pain is caused by the stimulation of free nerve endings. When the nerve endings are stimulated, sodiumenters the neuron, causing depolarization of the nerve and subsequent initiation of an action potential. The action potential is propagated down the nerve toward the central nervous system, which interprets this as pain. Benzocaine acts to inhibit the voltage-dependent sodium channels (VDSCs) on the neuron membrane, stopping the propagation of the action potential.8
Benzocaine undergoes ester hydrolysis to form 4-aminobenzoic acid, acetylation to form acetylbenzocaine, or N-hydroxylation to form benzocaine hydroxide. 4-aminobenzoic acid can be acetylated or acetylbenzocaine can undergo ester hydrolysis to form 4-acetaminobenzoic acid.9
References
1. Altenburg, A.; El-Haj, N.; Micheli, C.; Puttkammer, M.; AbdelNaser, M.B.; Zouboulis, C.C. The treatment of chronic recurrent oral aphthous ulcers. Dtsch. Arztebl. Int., 2014, 111(40), 665-673.
2. Hancock, P.J.; Epstein, J.B.; Sadler, G.R. Oral and dental management related to radiation therapy for head and neck cancer. J. Cancer Dent. Assoc., 2003, 69(9), 585-590.
3. Al Nadeesh, F.M.H. Synthesis and Characterization of Several Local Anaesthetics., M.Sc. Thesis, UniversitiTeknologi, Malaysia. 2013.
4. Demare, P.; Regla, I. Synthesis of two local anesthetics from toluene: An organic multistep synthesis in a project-oriented laboratory course. J. Chem. Edu., 2012, 89, 147.
5. Hanck DA, Nikitina E, McNulty MM, Fozzard HA, Lipkind GM, Sheets MF: Using lidocaine and benzocaine to link sodium channel molecular conformations to state-dependent antiarrhythmic drug affinity. Circ Res. 2009 Aug 28;105(5):492-9.
6. Carpenter, C.C.; Heinlein, J.A.; Sulzberger, M.B.; Baer, R.L. Scabies and pediculosis treated with benzyl benzoate, DDT benzocaine emulsion, including a comparison with other methods used at the U.S.N. hospital, Brooklyn, N. Y. J. Invest. Dermatol., 1946, 7, 93- 98.
7. Liebelt, E.L.; Shannon, M.W. Small doses, big problems: A selected review of highly toxic common medications. Pediatr. Emerg. Care, 1993, 9(5), 292-297.
8. Moore TJ, Walsh CS, Cohen MR: Reported adverse event cases of methemoglobinemia associated with benzocaine products. Arch Intern Med. 2004 Jun 14;164(11):1192-6.
9. Hartman NR, Mao JJ, Zhou H, Boyne MT 2nd, Wasserman AM, Taylor K, Racoosin JA, Patel V, Colatsky T: More methemoglobin is produced by benzocaine treatment than lidocaine treatment in human in vitro systems. Regul Toxicol Pharmacol. 2014 Oct;70(1):182-8.
Related articles And Qustion
See also
Lastest Price from Benzocaine manufacturers

US $0.00/kg2025-04-02
- CAS:
- 94-09-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $40.00-20.00/kg2025-03-07
- CAS:
- 94-09-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100tons