Baclofen: Chemical Properties, Applications, and Storage for Optimal Use
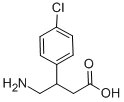
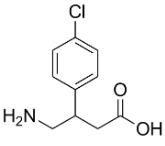
Baclofen, also known as β -4-chlorophenyl - γ - aminobutyric acid, is an agonist of the type B gamma-aminobutyric acid (GABAB) receptor and is sold under the brand names Kemstrom and Lioresal. This substance was initially approved for spastic states associated with neurological disorders and has recently become the primary treatment for AUD.

Figure 1 Characteristics of Baclofen
The function of Baclofen
Baclofen comes in two forms: oral formulations and intrathecal solutions, with the latter used to treat spastic states that do not respond to oral Baclofen. The recommended daily oral dose of Baclofen is 15 milligrams to 80 milligrams, starting from 15 milligrams per day and gradually increasing by 5 milligrams every three days. Despite significant individual differences in pharmacokinetics, baclofen is rapidly absorbed from the gastrointestinal tract after oral administration. Up to 80% of oral doses are excreted through urine, with limited liver metabolism, making it a useful medication for patients with liver dysfunction, and careful evaluation of kidney function should be conducted before taking Baclofen. In addition, the half-life of Baclofen is very short, only 3 to 4 hours, and it can be quickly cleared from the bloodstream. Therefore, Baclofen needs to be taken 3 or 4 times a day to maintain its therapeutic effect.
Clinical research
Preclinical studies have found that Baclofen can reduce the acquisition, maintenance, and recovery of drinking behavior in rats. Evaluating the impact on recovery by modifying the so-called 'alcohol deprivation effect', a rodent model used to study relapse behavior, refers to a temporary increase in voluntary alcohol consumption after a period of forced abstinence in a free choice experiment. In addition, Baclofen has been shown to dose-dependently reduce the number of lever reactions to alcohol and the amount of self-administered alcohol in rats under operational self-administration conditions. In operational self-management experiments, a certain amount of "work" is required to obtain alcohol, which has predictive validity for human alcohol cravings and provides measurements of alcohol consumption and alcohol-enhancing properties. Therefore, it was found that Baclofen is more effective and efficient for rats seeking and consuming large amounts of alcohol.[1]
A series of case reports have found that the administration of Baclofen significantly reduced alcohol intake in individuals affected by AUD. One of the reports described the personal medical history of a French doctor who used Baclofen to explore the original dose curve to treat his own AUD. He tested oral Baclofen, starting from a "regular" dose of 30 milligrams per day and increasing the daily dose to 270 milligrams five weeks later, experiencing "completely drug-induced alcohol craving suppression" and relieving comorbid anxiety.
Although numerous case reports, case series, and open-label trials have been published demonstrating the effectiveness of baclofen in patients with AUD, the randomized controlled trials (RCTs) conducted so far have yielded conflicting results. The diversity of research results has stimulated discussions on potential moderating factors of effectiveness. Among them, the severity of alcohol dependence, the dosage of Baclofen, and comorbidity anxiety have been discussed to alleviate the impact of Baclofen on alcohol consumption.
Safety of Baclofen
Although the data on the efficacy of baclofen in AUD is mixed, they are consistent in terms of safety when used as recommended. For decades, baclofen has been widely used to treat spastic states, from which extensive information about its safety has been obtained. Most adverse events are not serious, dose-related, and short-lived. According to reports, sedation, drowsiness, weakness, dizziness, and psychological disorders are the most common adverse events. The administration of low-dose diclofenac is considered safe for people affected by AUD, while sudden interruption and withdrawal of higher doses of diclofenac may lead to serious adverse reactions. Although such events are rare, the lack of known adverse events may be due to the small sample size and low dose of baclofen used in existing trials.[2]
References:
[1] PER ERTZGAARD A C Claudia Campo. Efficacy and safety of oral baclofen in the management of spasticity: A rationale for intrathecal baclofen.[J]. Journal of Rehabilitation Medicine, 2017, 49 3. DOI:10.2340/16501977-2211.[2] T H LEE. Baclofen intoxication: report of four cases and review of the literature.[J]. Clinical Neuropharmacology, 1992, 15 1.
Related articles And Qustion
See also
Lastest Price from Baclofen manufacturers

US $0.00/KG2025-09-25
- CAS:
- 1134-47-0
- Min. Order:
- 1KG
- Purity:
- ≥99.9%(HPLC)
- Supply Ability:
- 10000KGS

US $0.00-0.00/KG2025-09-22
- CAS:
- 1134-47-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1T