Avobenzone: Understanding Its Tautomerism, Photodecomposition, and Structure-Activity Relationship
General Description
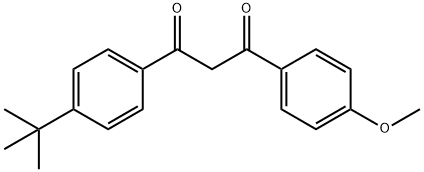
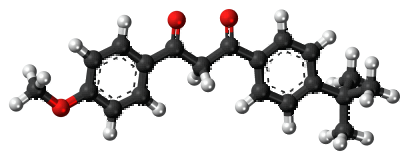
Avobenzone exists as a dynamic equilibrium between its enol and keto forms, with the enol form exhibiting two isomeric forms. The methoxy group present in the enol form facilitates strong H-bonding and resonance-assisted delocalization. Spectroscopy analysis shows distinct bands for each form, with phosphorescence observed for the enol form and strong phosphorescence in the range of 410-450 nm for the keto form. Photodecomposition occurs upon UV light exposure, with the complete degradation of Avobenzone being observed after 100 hours. Electron-withdrawing groups on benzene ring A increase photostability, while electron-releasing groups destabilize it. Modifications that block the diketo group, such as methylation of the acidic hydrogen at position 'b,' also enhance photostability.

Figure 1. Avobenzone
Tautomerism
Avobenzone is a compound that exists in solution as a dynamic equilibrium between its enol and keto forms. The enol form can exist in two isomeric forms, while the keto form has a butterfly-like structure. The presence of a methoxy group facilitates delocalization of the enolic form, leading to resonance-assisted H-bonding in the chelated enol form. This combination of pi delocalization and H-bond formation results in the formation of strong H-bonds. Under certain conditions, such as exposure to light, the enol form can undergo photolysis and convert to the keto form. However, in the absence of light, the keto form reverts back to the enol form. This process is reversible. When analyzing Avobenzone using spectroscopy, two distinct bands are observed. The enol form produces a band at 355 nm, while the keto form in cyclohexane produces a band at 265 nm. In more polar solvents, the wavelength of the enol band shifts to higher values, such as 356 nm in ethyl acetate, 363 nm in DMSO, and 358 nm in methanol. The enol form exhibits phosphorescence with a duration of 30 milliseconds. When diluted in ethyl acetate, Avobenzone fluoresces with a peak wavelength at 405 nm, a maximum yield of 0.01, and a fluorescence lifespan of 13 picoseconds. On the other hand, the keto form shows strong phosphorescence in the range of 410-450 nm but is only marginally luminous. The triplet state, which is responsible for phosphorescence, does not exhibit an EPR (electron paramagnetic resonance) signal. The photoinstability of Avobenzone is attributed to the long-lived triplet state of the keto form. 1
Photodecomposition
The photodecomposition of Avobenzone involves the excitation of an electron from the lowest energy state to the singlet-excited state upon absorption of UV light. This excited electron can either transition to the excited triplet state or return to the ground state, leading to phosphorescence, heat production, or further photodegradation processes. This photodecomposition process can cause photoallergic reactions in the skin. After exposure to a mercury lamp for 100 hours (185-400 nm), the complete photodecomposition of Avobenzone in cyclohexane was observed. Analysis using GC-MS revealed three degradation products: p-methoxy benzoic acid, p-tert-butyl benzoic acid, and t-butylbenzene. Harsh photochemical conditions contributed to the degradation of some principal photoproducts. The production of benzoic acids can be attributed to the breaking of one of the two C-C bonds near the C=O of the keto form. This photodegradation process occurs in the triplet state. Photodecomposition is a complex process that generates various compounds with preferential absorption at wavelengths below 260 nm. The ground-state enol and singlet or triplet oxygen produced by the triplet keto form can combine to form oxygenated molecules. Additionally, the triplet keto form can also degrade the enol form during photolysis. 2
Structure-Activity Relationship
Avobenzone is a chemical compound that exists in two forms: the unstable keto form and the more stable enol form. The instability of the keto form is responsible for its photodegradation. However, it can readily convert into the enol form in solution. A study conducted by Raghothama et al investigated the impact of different functional groups on two benzene rings (referred to as rings A and B) of avobenzone. The study found that electron-withdrawing groups on benzene ring A, such as carboxylic acid and methyl carboxylate, increased the photostability of avobenzone. On the other hand, electron-donating groups on the same benzene ring destabilized avobenzone. Another study by Miranda et al showed that methylation of the acidic hydrogen at position 'b' also enhanced the photostability of avobenzone. The explanation for the increased stability is that these modifications block the diketo group, which is responsible for the photodegradation of avobenzone. Additionally, the presence of a butylated benzene ring (ring B) is crucial for the activity of avobenzone as it helps trap the free radicals generated by sunlight exposure. Overall, the study indicates that electron-withdrawing groups enhance the stability of avobenzone towards photodegradation, while electron-releasing groups may destabilize it. Future research could explore the effects of other electron-withdrawing groups like nitro, cyano, and carbonyl compounds on the stability of avobenzone. 3
Reference
1. Gholap AD, Sayyad SF, Hatvate NT, et al. Drug Delivery Strategies for Avobenzone: A Case Study of Photostabilization. Pharmaceutics, 2023, 15(3): 1008.
2. Dutta P. In Light of Exposure: Understanding Avobenzone Part I, Characterization, 2022.
3. Paris C, Lhiaubet-Vallet V, Jiménez O, Trullas C, Miranda Má. A Blocked Diketo Form of Avobenzone: Photostability, Photosensitizing Properties and Triplet Quenching by a Triazine-Derived UVB-Filter. Photochem. Photobiol. 2009, 85, 178–184.
Related articles And Qustion
See also
Lastest Price from Avobenzone manufacturers
US $0.00/kg2025-11-05
- CAS:
- 70356-09-1
- Min. Order:
- 25kg
- Purity:
- 0.99
- Supply Ability:
- /

US $1.50/g2025-06-24
- CAS:
- 70356-09-1
- Min. Order:
- 1g
- Purity:
- 99.0% Min
- Supply Ability:
- 10 Tons