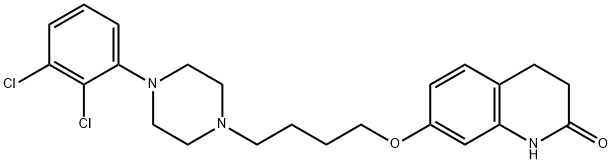
Aripiprazole: Pharmacodynamics, Pharmacokinetics and Adverse Effects
General Description
Aripiprazole is an atypical antipsychotic characterized by its high affinity for dopamine receptors and its unique mechanism as a partial agonist at D2 and 5-HT1A receptors, while acting as an antagonist at 5-HT2A receptors. This stabilizing effect allows it to modulate symptoms of schizophrenia and bipolar disorder with a lower risk of extrapyramidal symptoms, sedation, and weight gain compared to typical antipsychotics. Its pharmacokinetics vary by administration route, showing high bioavailability and a long half-life. While generally well-tolerated, aripiprazole may cause adverse effects such as metabolic changes and, rarely, neuroleptic malignant syndrome. Monitoring is recommended.

Figure 1. Aripiprazole
Pharmacodynamics
Receptor Binding Profile
Aripiprazole's pharmacodynamics are distinguished by its selective receptor binding profile. Aripiprazole exhibits a high affinity for dopamine receptors, with a Ki value of 0.74 nmol/L, indicating a stronger binding than many other antipsychotic drugs, except for risperidone. In comparison, typical antipsychotics like haloperidol show a lower affinity for dopamine receptors, with a Ki value of 1.4 nmol/L. Aripiprazole's pronounced binding to dopamine receptors contributes to its efficacy in treating schizophrenia and bipolar disorder. Additionally, aripiprazole functions as a partial agonist at the 5-HT1A receptor and an antagonist at the 5-HT2A receptor, with Ki values of 5 and 9 nmol/L, respectively. This dual activity modulates serotonin levels, which is crucial for its therapeutic effects. Moreover, aripiprazole has a lower affinity for opioid, norepinephrine, serotonin, benzodiazepine, GABA, NMDA, and muscarinic receptors, and binds strongly to histamine H1 and adrenergic α1a and α1β receptors, which contributes to both its therapeutic efficacy and its side effect profile.
Dopamine–5-HT System Stabilization
Aripiprazole's mechanism of action involves its role as a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G-protein-coupled state, aripiprazole can block the receptor in the presence of excessive dopamine, while stimulating the receptor when dopamine levels are normal. This unique stabilization mechanism differentiates aripiprazole from other antipsychotics, such as haloperidol, which acts solely as a pure dopamine antagonist. Consequently, aripiprazole has a reduced potential for extrapyramidal symptoms (EPS), sedation, and elevated serum prolactin levels compared to many other antipsychotics. This pharmacodynamic profile allows aripiprazole to effectively manage symptoms with a favorable side effect profile. 1
Pharmacokinetics
Absorption
Aripiprazole exhibits distinct pharmacokinetic properties depending on its route of administration. After intravenous administration, aripiprazole extensively binds to plasma proteins and has a large steady-state volume of distribution of 4.9 L/kg, indicating significant distribution beyond the vascular system. The half-life of aripiprazole in this context is approximately 58 hours, allowing for prolonged therapeutic effects. In contrast, following intramuscular administration, aripiprazole demonstrates linear and dose-proportional pharmacokinetics. The bioavailability of intramuscular aripiprazole is notably high at 98%, compared to 87% for the oral form, and achieves peak plasma concentrations within 0.5 hours. This route results in higher plasma concentrations and faster absorption compared to oral administration. 2
Metabolism and Special Considerations
Aripiprazole is primarily metabolized in the liver through various pathways including N-dealkylation, hydroxylation, and dehydrogenation, with CYP3A4 and CYP2D6 enzymes playing significant roles. Its major active metabolite, dehydroaripiprazole, reaches steady-state systemic exposure at about 40% of the parent drug. Excretion of aripiprazole occurs through both urine and feces, with minimal unchanged drug found in these excreta. Importantly, aripiprazole's pharmacokinetics are relatively stable across hepatic or renal impairments, and it does not significantly affect or get affected by the pharmacokinetics of several other drugs. Adjustments in aripiprazole dosing may be necessary when interacting with CYP inhibitors or inducers. 2
Adverse Effects
Aripiprazole, an atypical antipsychotic, has a generally favorable safety profile with several notable adverse effects. Unlike some antipsychotics, aripiprazole causes minimal sedation and has a low risk of affecting the QTc interval, though rare cases of supraventricular tachycardia have been reported. Compared to haloperidol, aripiprazole is associated with a lower incidence of tardive dyskinesia. It also shows a low propensity for causing weight gain, cardiovascular abnormalities, hyperprolactinemia, hypercholesterolemia, or glucose dysregulation. While aripiprazole has minimal hematologic effects, some reports link it to atypical neuroleptic malignant syndrome and classic neuroleptic malignant syndrome. Additionally, aripiprazole has been associated with dermatological issues such as acanthosis nigricans, indicative of insulin resistance, and may cause asymptomatic hyperamylasemia and pancreatitis. Overall, while aripiprazole is generally well-tolerated, monitoring for these adverse effects is advisable. 1
References:
[1] MARIJA JOVANOVI? B M Katarina Vu?i?evi?. Understanding variability in the pharmacokinetics of atypical antipsychotics - focus on clozapine, olanzapine and aripiprazole population models.[J]. Drug Metabolism Reviews, 2020, 52 1. DOI:10.1080/03602532.2020.1717517.See also
Lastest Price from Aripiprazole manufacturers

US $5.00-0.50/KG2025-05-29
- CAS:
- 129722-12-9
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $0.00/Kg/Bag2025-04-21
- CAS:
- 129722-12-9
- Min. Order:
- 1KG
- Purity:
- 98.0%~102.0%; USP39
- Supply Ability:
- 1000kg/month