Applications of Triphenylphosphine oxide
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3. This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds.
Properties
Triphenylphosphine oxide (Ph3PO) has a polar P+–O− bond (dipole moment: 4.31 D),whose negatively charged oxygen atom can bind to protons, metals, and other electrophiles. Therefore, Ph3PO serves as an effective nucleophilic (Lewis base) catalyst or promoter, a ligand for metal catalysts, or a scavenger of metals or electrophiles. Despite the low pKa of the conjugate acid (−2.1), Ph3PO complexes with a variety of hydrogen-bond donors such as water, alcohols, phenols, carboxylic acids, and amides to aid their crystallization.
Applications
As a Catalyst or Promoter. Ph3PO promotes allylation of aldehydes (eq 1)or N-acylhydrazones (eq 2)with allyltrichlorosilanes. Ph3PO coordinates to allyltrichlorosilanes to generate hypervalent silicon compounds as reactive intermediates. In the former case, addition of a tetrabutylammonium salt effectively shortens the reaction time.
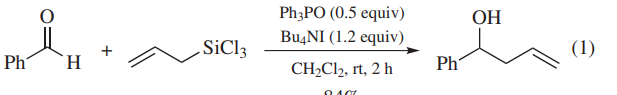
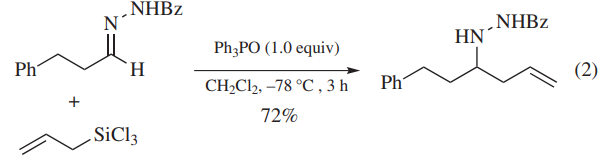
A catalytic amount of Ph3PO activates trichlorosilane to facilitate conjugate reduction of α,β-unsaturated ketones.Trichlorosilyl enol ethers are formed as the primary product, and the corresponding saturated ketones are obtained after hydrolysis. When the conjugate reduction is conducted in the presence of an aldehyde, the sequential aldol reaction (reductive aldol reaction) takes place.
As a Cocatalyst for Metal-catalyzed Processes. Ph3PO serves as an effective cocatalyst in chiral Lewis acid-catalyzed enantioselective cyanosilylations of aldehydes or ketones with trimethylsilyl cyanide. In this chemistry, Ph3PO reacts with trimethylsilyl cyanide to generate an isocyanide species Ph3P(OSiMe3)(N=C:) as a reactive intermediate.
As a Stabilizing Ligand for Metal Catalysts. The presence of a catalytic amount of Ph3PO suppresses decomposition of palladium catalysts for the cross-coupling reaction of potassium organosilanolates with aryl halides.The improved yield and reproducibility are obtained without the rigorous exclusion of oxygen.
As a Scavenger. Ph3PO may be employed to remove ruthenium by-products generated during olefin metathesis reactions with Grubbs catalysts.Thus, treatment of the crude reaction products with Ph3PO followed by filtration through silica gel is a practical and effective method to remove colored ruthenium byproducts.
reference
1.Gilkerson, W. R.; Ezell, J. B., J. Am. Chem. Soc. 1967, 89, 808
2.Short, J. D.; Attenoux, S.; Berrisford, D. J., Tetrahedron Lett. 1997, 38, 2351.
3. Ogawa, C.; Konishi, H.; Sugiura, M.; Kobayashi, S., Org. Biomol. Chem. 2004, 2, 446.
4. Sugiura, M.; Sato, N.; Kotani, S.; Nakajima, M., Chem. Commun. 2008,4309.
You may like
See also
Lastest Price from Triphenylphosphine oxide manufacturers

US $0.00/KG2025-04-21
- CAS:
- 791-28-6
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month

US $1.00/kg2025-04-21
- CAS:
- 791-28-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 mt
![174063-87-7 1,4-Bis-[4-(3-acryloyloxypropyloxy)benzoyloxy]-2-methylbenzene; Preparation](https://www.chemicalbook.com/NewsImg/2019-11-7/2019117142424284.jpg)