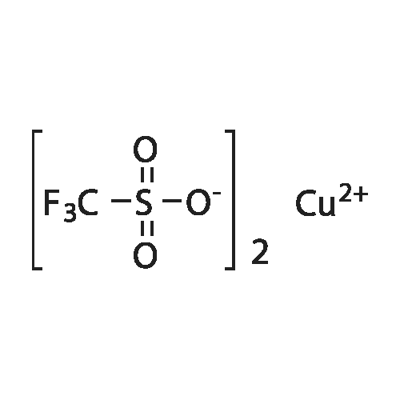
Applications of Copper(Ⅱ) Trifluoromethanesulfonate
Copper(Ⅱ) Trifluoromethanesulfonate is the copper(II) salt of trifluoromethanesulfonic acid which has a chemical formula of Cu(OSO2CF3)2. This substance, first reported in 1972, is a powerful Lewis acid. Copper(II) trifluoromethanesulfonate is a mild lewis acid. It is used as catalyst which promotes dehydration of alcohols and diols to alkenes at ambient temperatures. Copper(Ⅱ) Trifluoromethanesulfonate is widely used to generate carbenoid species from diazo esters and ketones, via in situ reduction to the Cu(I) species. Copper(Ⅱ) Trifluoromethanesulfonate is also promotes the reaction between diazo esters and imines to give aziridines. It catalyzes syn-selective aldol condensation of (Z)-silyl enol ethers with aldehydes, Friedel-Crafts alkylation, acylation reactions of aromatics and addition of trimethylsilyl cyanide to carbonyl compounds.
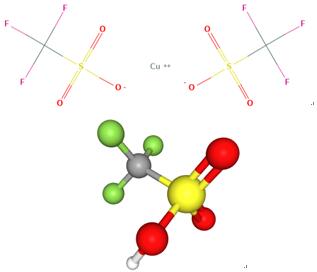
Fig 1. Chemical structure formula and three-dimensional structure of COPPER(II) TRIFLUOROMETHANESULFONATE
Copper(Ⅱ) Trifluoromethanesulfonate is used as a catalyst in several organic reactions, such as the Diels-Alder reaction and cyclopropanation reactions (much like rhodium(II) acetate). Copper(II) trifluoromethanesulfonate acts as a catalyst in Diels-Alder reaction and cyclopropanation reactions. Copper(II) trifluoromethanesulfonate is also used as a reagent for oligosaccharide synthesis. Copper(II) trifluoromethanesulfonate acts as a Lewis acid. It is also employed in the dimerization of ketone enolates and tetramethylsilane enol ethers. Copper(II) trifluoromethanesulfonate is used to prepare cis vinylic sulfones and oxazoles from alkynyl sulfones and ketones respectively. Further, it plays an important role for cyclization of dienolates. In addition to this, Copper(II) trifluoromethanesulfonate is useful for dehydration of alcohols[1].Both intermolecular and intramolecular oxidative coupling reactions can be effected using Copper(II) trifluoromethanesulfonate. Examples of dimerization include one-pot syntheses of 1,4-diketones from ketone enolates or from silyl enol ethers (eqs 1 and 2), and coupling of allylstannanes with TMS-enol ethers to give g,d-unsaturated ketones in good to moderate yields (eq 3). Other copper(II) or tin(IV) catalysts can also be used with allylstannanes. The regiochemistry depends on both the substrate and the catalyst[2].
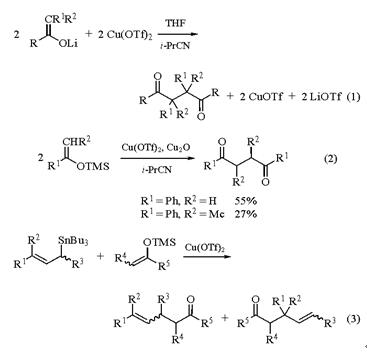
Fig 2. Intermolecular and intramolecular oxidative coupling reactions
Copper(II) trifluoromethanesulfonate is the reagent of choice for intramolecular cyclization of b,g-unsaturated diazo ketones to cyclopentenones and for intramolecular cyclopropanation of g,d-unsaturated diazo ketones. Angularly functionalized polycyclic systems may be prepared from b,g-unsaturated diazo ketones by a vinylogous Wolff rearrangement in the presence of copper(II) trifluoromethanesulfonate. It is a useful reagent for orthoester homologation via dialkoxycarbenium ions and for oxazole formation by reaction of ketocarbenes (via diazo esters/Copper(II) trifluoromethanesulfonate with nitriles. With unsaturated nitriles, the nitrile group is selectively attacked. copper(I) trifluoromethanesulfonate (CuOTf) was first prepared as a solution in acetonitrile by synproportionation of Copper(II) Trifluoromethanesulfonate with copper(0).
References
[1] Jha, M.; Shelke, G. M.; Pericherla, K.; Kumar, A. Microwave assisted copper triflate-catalyzed rapid hydration of aryl acetylenes. Tetrahedron Lett. 2014, 55 (34), 4814-4816.
[2] Kobayashi, Y.; Taguchi, T.; Tokuno, E. TL 1977, 3741.
You may like
Related articles And Qustion
See also
Lastest Price from COPPER(II) TRIFLUOROMETHANESULFONATE manufacturers

US $10.00/KG2025-04-21
- CAS:
- 34946-82-2
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00-0.00/KG2025-04-15
- CAS:
- 34946-82-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg