Application and Preparation of Benzophenone
General description
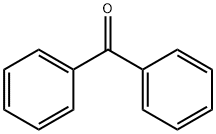
The Benzophenone, with the CAS No: 119-61-9, is also known as Diphenylmethanone, Benzoylbenzene, α-Oxodiphenylmethane. It appears as white solid with a flowery odor and may float or sink in water, insoluble in water, soluble in ethanol, ether and chlorofor, mmelting point: 47~49℃. This chemical's molecular formula is C13H10O and molecular weight is 182.22. Benzophenone is the simplest member of the class of benzophenones, being formaldehyde in which both hydrogens are replaced by phenyl groups (Figure 1). It has a role as a photosensitizing agent and a plant metabolite. Benzophenone is a common fine chemical intermediate and additive, which is widely used in pesticides, pharmaceuticals, electronic chemicals and many other fields [1]. In the pharmaceutical industry, it is used to produce dicyclohexylpiperidine, benzotropine hydrobromide, diphenhydramine hydrochloride, etc. Because it can give essence a sweet smell, it is used in all kinds of perfume and essence.
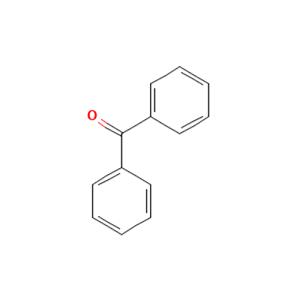
Figure 1. The molecular formula of Benzophenone
Application of Benzophenone
With the further research of benzophenone, its application scope is more and more extensive, mainly including the following applications.
Photoinitiator
Photoinitiator is also called light curing agent and photosensitizer. Benzophenone has simple molecular structure and is commonly used as hydrogen capturing photoinitiator [2]. Its advantages are fast curing speed, wide matching range, good thermal stability, high quantum efficiency, good solubility, low price, etc. It is now the photoinitiator with the largest output used in the following polymerization reactions: unsaturated prepolymers, such as acrylate, with single or multifunctional vinyl monomers. Its disadvantages are that the residual photoinitiator in the process of use will lead to the aging and yellowing of the cured coating, and toxic odor will also appear, which limits the application value. With the deepening of research, many inventors carried out chemical reaction experiments on the basis of compounds with simple structure and small molecular weight, and obtained multi-functional macromolecular compounds as photoinitiators to improve the optical properties of the compounds, so that they have better optical activity, thus overcoming the above shortcomings.
UV absorber
Benzophenone compounds are currently the largest UV absorbers, which can absorb 290-400nm UV absorption light and some visible light, so they are widely used in coatings, resins, cosmetics and other industries [3]. For example, most sunscreens on the market contain benzophenone compounds, which can reduce the damage of UVA and UVB to the skin, and have the advantages of safety and independence.
Pharmaceutical intermediate
Benzophenone can be used as the basis for the synthesis of important drug frameworks, and diphenyl carbinols can be synthesized by reduction reaction. For example, diphenhydramine in antihistamines is an anti vertigo drug, in which the intermediate is benzophenone, and the synthesis steps are as follows (Figure 2). In addition, it is used in the pharmaceutical industry to produce dicyclohexylpiperidine, benzotropine hydrobromide, diphenhydramine hydrochloride, etc [4].
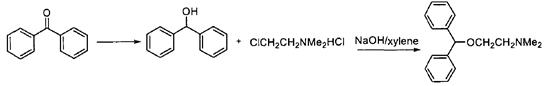
Figure 2. Synthesis of Diphenhydramine
Indicator
In synthesis experiments, it is often used as an indicator for treating solvents such as benzene, toluene, xylene, tetrahydrofuran, acetonitrile, etc [5]. for example, take a small amount of acetonitrile solution into a beaker, add benzophenone, and stir it slowly. If the color of the solution turns blue, it indicates that there is water in the acetonitrile solution, and carry out post-treatment according to the needs. The principle is that benzophenone is reduced to produce sodium benzophenone under the action of sodium. This free radical is relatively stable, while water and oxygen can quench free radicals, so it can indirectly indicate the anaerobic condition.
Stabilizer
Benzophenone compounds can be used as stabilizers for polycarbonate, acrylic resin, vinyl plastics and other polymer materials [6]. The principle is that benzophenone compounds can absorb ultraviolet rays in sunlight, so as to prevent ultraviolet rays from damaging the dyeing structure, play a protective role, and then improve the durability of textiles.
Preparation
At present, the main preparation methods of benzophenone include benzoyl chloride method, benzoic acid method, trichlorotoluene method, phosgene method, benzyl chloride method, carbon tetrachloride method and format reagent[7-10].
Benzoyl chloride method
This method uses anhydrous benzene and benzoyl chloride as raw materials, the molar ratio of materials is 10:1, anhydrous aluminum chloride as catalyst, heating reflux to prepare benzophenone compounds (Scheme 1) .
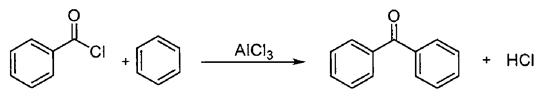
Scheme 1. Synthesis of benzophenone by Benzoyl chloride method
Benzoyl chloride method has the advantages of simple process, short production cycle, high production efficiency and convenient industrial production. The disadvantages of this method are that benzoyl chloride has high price, raw materials need pre distillation treatment, inconvenient transportation and high risk, resulting in high cost, large amount of aluminum chloride is required in the production process and it is difficult to recover, and the by-product HCl has serious corrosion to the equipment.
Benzoic acid method
In this method, benzoic acid and m-xylene are used as raw materials, equal molar Lewis acid is used as catalyst, and benzophenone compounds are prepared by heating reflux (Scheme 2).
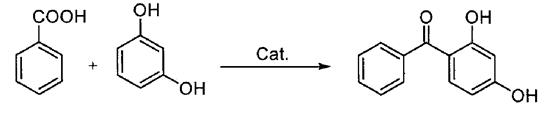
Scheme 2. Synthesis of benzophenone by Benzoic acid method
Trichlorotoluene method
This method uses trichlorotoluene and m-xylene as raw materials to prepare benzophenone compounds. Since trichlorotoluene is insoluble in water, an excess of catalyst is required. In addition, catalyst recovery and product purification are also more difficult.
Phosgene method
In phosgene process, benzene and phosgene are used as raw materials, anhydrous aluminum chloride is used as catalyst, Friedel-crafts alkylation reaction is carried out to obtain intermediate products, and then benzophenone compounds are obtained by hydrolysis (Scheme 3).
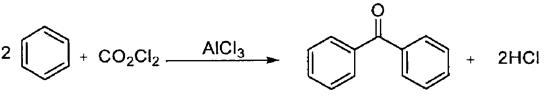
Scheme 3. Synthesis of benzophenone by Phosgene method
Phosgene method has the advantages of low raw material cost, less by-products, less industrial three wastes, mild production process conditions and high product yield. However, it has high requirements for equipment, strict operation requirements, and phosgene is a severe poison. Moreover, phosgene is a severe poison, which will bring potential dangers to the environment and safety as a raw material.
Benzyl chloride method
In this method, benzyl chloride and benzene are used to produce diphenylmethane under the action of aluminum trichloride catalyst, and then benzophenone is prepared by nitric acid oxidation (Scheme 4). The raw materials of this method are cheap and easy to obtain, the requirements for production equipment are low, and the product yield is high, but the process is complex, the by-product is polyphenyl compounds, and the reaction temperature is high.
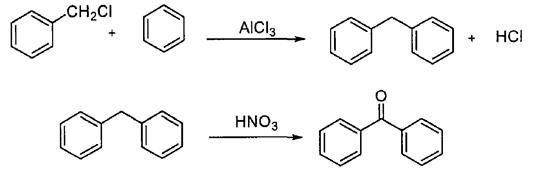
Scheme 4. Synthesis of benzophenone by Benzyl chloride method
Carbon tetrachloride method
In the method, benzene and carbon tetrachloride are catalyzed by anhydrous aluminum chloride to obtain diphenyldichloromethane, which is then hydrolyzed to obtain benzophenone compounds (Scheme 5). This method has the advantages of simple process route, high product yield and less by-products, which can reduce the environmental pollution caused by the separation process.
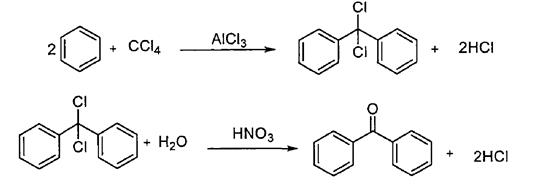
Scheme 5. Synthesis of benzophenone by Carbon tetrachloride method
Grignard reagent method
Grignard reagent method utilizes benzoyl chloride and Grignard reagent as raw materials to prepare benzophenone compounds at low temperature (Scheme 6). The advantages of this method are simple process route, high product yield and less by-products, but the raw material price is high and it is easy to pollute the environment, resulting in high generation cost.
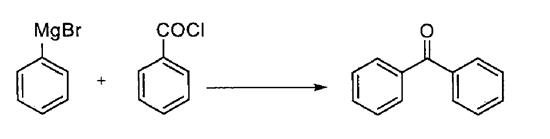
Scheme 6. Synthesis of benzophenone by Grignard reagent method
MeSH Pharmacological Classification[11]
As a photosensitizing agent, benzophenone are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms.
Toxicity[11]
HUMAN EXPOSURE AND TOXICITY: There is limited information on the toxic effects of benzophenone in humans published. It showed little activity in the hormone-responsive reporter assay tested in various cell lines. ANIMAL STUDIES: Benzophenone showed no estrogenic activity, however, after irradiating an aqueous solution of Benzophenone with UV or sunlight, it can be converted into ring-hydroxylated derivatives (3-hydroxy BP (BP-3OH) and 4-hydroxyBP (BP-4OH)) that have estrogenic activity.
References
1. Wang Shuqing, Sun Dongbing, Xia Ming, Yao Shuyu. Study on the synthesis of light stabilizer benzophenone[J].Chemical World,2010,51(01):43-45.
2. George B , Dhamodharan R. A study of the photopolymerization kinetics of methyl methacrylate using novel benzophenone initiators[J]. Polymer International, 2001, 50(8):897-905.
3. Ning Peisen, Wang Kechang, Ding Zhuming. The Situation and Developing Trend of Benzophenone Derivatives Ultraviolent Absorber[J]. Plastics Additives, 2008(2):7-12.
4. Zhang A , Zhang Z , Gong Y . Grafting of mah and polypropylene in supercritical CO2 medium[J]. Acta Polymerica Sinica, 2004, 92(2):292-295.
5. Kanerva - Rustemeyer L, Elsner P, John SM, Maibach HI (eds). Kanerva's Occupational Dermatology, 2nd Ed. Berlin: Springer-Verlag, 2012., p. 1755.
6. Zhu Bairan. Synthesis process of quinoline and benzophenone[D].Yangzhou University,2020.
7. Kishore D,Rodrigues A E.Catalysis Communi-cations[J],2009,(10):1212-1215.
8. Zhou B D, Wei R R, Li J L, et al. Synthesis and antitumor activity of benzophenone compound[J]. Journal of Asian Natural Products Research, 2022, 24(2): 170-178.
9. Fletcher P, Marlow W. Synthesis of benzophenones: anomalous Friedel–Crafts reactions[J]. Journal of the Chemical Society C: Organic, 1970 (7): 937-939.
10. Xiangxiang YIN, Lei W, Jun NIE, etal. Synthesis and Properties of a Novel Benzophenone Photoinitiator[J]. IMAGING SCIENCE AND PHOTOCHEMISTRY, 2018, 36(2): 200.
11. National Center for Biotechnology Information. "PubChem Compound Summary for CID 3102, Benzophenone" PubChem,
Related articles And Qustion
See also
Lastest Price from Benzophenone manufacturers

US $5.00-2.00/KG2025-06-10
- CAS:
- 119-61-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000kg

US $0.00/KG2025-04-21
- CAS:
- 119-61-9
- Min. Order:
- 25KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month