An excellent hydrogen energy carrier--methylcyclohexane
Introduction
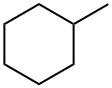
Among the cycloalkanes, methylcyclohexane (MCH) is the simplest alkylated cyclohexane and has been chosen as the model compound for surrogate fuels by many studies[1]. In addition to being a conventional endothermic hydrogen fuel, it can also provide a heat sink up to 2.19 MJ/kg through dehydrogenation reactions, thereby meeting the requirement to cool jet engines.
Properties
Methylcyclohexane (MCH) is regarded as an excellent hydrogen energy carrier due to its reasonable theoretical hydrogen storage content (6.22wt%) and physicochemical performance[2]. The observed ignition delay times can be summarized by the relation: MCH>BCH>CH. The rate at which chain-carriers appear to help explain the differences among CH, MCH and BCH.

Picture 1 Methylcyclohexane liquid
Sructure
Methyleyclohexane has the simplest structure among all substituted cyclohexanes. Methyleyclohexane may have its methyl group in either a polar or an equatorial position[3]. By studying a model of this molecule. it is found that steric interactions arise which arc similar to those of w-paraffins in their various configurations. Polar methyleyclohexane has two similar interactions whereas the equatorial form has no strain of this type. Therefore, the energy of the former is greater than that of the latter by approximately twice the w-butane value. In the trans-1,4 compound the energy difference is clearly twice that in methyleyclohexane, 4a or 3.6 kcal./mole.
Application as endothermic fuels
Endothermic fuels such as methylcyclohexane (MCH) have been proposed to cool supersonic vehicles from the intense heat developed during highspeed flight, not only by sensible and latent heat uptake, but also through the catalytic, endothermic decomposition of the fuel [1]. MCH, in the presence of a catalyst, can be endothermically (~ 205 kJ/mol) dehydrogenated to toluene and hydrogen. As much as 48% of MCH's total heat-absorbing capacity occurs through this decomposition characteristic. Furthermore, the toluene and hydrogen produced are the fuels consumed within the aircraft combustor[4].
Application as hydrogen energy carrier
The gravimetric H2 density is highest in liquid H2, followed by NH3, while NH3 shows the highest volumetric density. MCH has both the lowest gravimetric and volumetric H2 densities, leading to the largest tank-to-store/transport ratio for the same amount of H2[5]. In addition, in terms of auto-ignition temperature, NH3 has the highest temperature of 874 K, followed by liquid H2 (818 K), while MCH shows the lowest (809 K). To store and transport MCH and NH3, currently available infrastructure and regulations are considered sufficient, while liquid H2 still needs further development and regulation improvement. From the utilization standpoint, liquid H2 seems to be ideal for utilization, demanding very high H2 purity, such as in a fuel cell. In contrast, in the toluene-MCH cycle, H2 must be released through dehydrogenation, and if high purity of H2 is demanded, further purification must be introduced, leading to lower total energy efficiency. NH3 has a relatively higher flexibility in utilization, as it can be used directly or be decomposed to release its H2. Regarding the challenges, three storage methods face similar problems in large energy consumption, although in different ways.
Liquid H2 faces a big problem related to energy consumption during liquefaction. In addition, it also has the challenge of boil-off during storage. Similarly, MCH also demands high energy input for dehydrogenation, to release H2 and storage size[1]. Finally, the main problem for NH3 is the huge energy input in its synthesis from N2 production. In addition, NH3 is identical to fertilizer; its utilization as an energy source is feared to compete with it. Therefore, a massive adoption of NH3 in energy sector would also influence the agricultural sector, especially in terms of economics.
Reference
1 Liu Y, Ding J, Han K L. Molecular dynamics simulation of the high-temperature pyrolysis of methylcyclohexane[J]. Fuel, 2018, 217: 185-192.
2 Hong Z, Lam K Y, Davidson D F, et al. A comparative study of the oxidation characteristics of cyclohexane, methylcyclohexane, and n-butylcyclohexane at high temperatures[J]. Combustion and Flame, 2011, 158(8): 1456-1468.
3 Beckett C W, Pitzer K S, Spitzer R. The thermodynamic properties and molecular structure of cyclohexane, methylcyclohexane, ethylcyclohexane and the seven dimethylcyclohexanes1[J]. Journal of the American Chemical Society, 1947, 69(10): 2488-2495.
4 Pitz W J, Naik C V, Mhaoldúin T N, et al. Modeling and experimental investigation of methylcyclohexane ignition in a rapid compression machine[J]. Proceedings of the combustion institute, 2007, 31(1): 267-275.
5 Zeppieri S, Brezinsky K, Glassman I. Pyrolysis studies of methylcyclohexane and oxidation studies of methylcyclohexane and methylcyclohexane/toluene blends[J]. Combustion and flame, 1997, 108(3): 266-286.
See also
Lastest Price from Methylcyclohexane manufacturers

US $0.00/KG2025-04-21
- CAS:
- 108-87-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $0.00/kg2025-03-07
- CAS:
- 108-87-2
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons