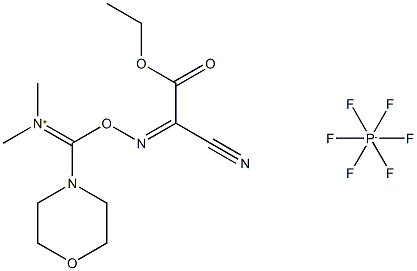
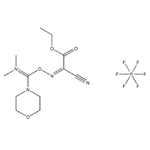
An efficient Coupling Reagent: COMU
Description
The methodology for peptide bond formation is undergoing a continuous evolution where the main actors are being renewed. In recent years, coupling reagents based on the Oxyma scaffold, such as the uronium salt COMU, has been a groundbreaking contribution to the field. The advantages of COMU over classic benzotriazole‐based reagents (HATU, HBTU, HCTU, TBTU) were proven in terms of solubility and coupling efficiency in bulky junctions in researchers.
Structure and advantages
COMU is a more efficient alternative to classical benzotriazole immonium salts regarding racemization suppression, coupling effectiveness, stability, and solubility. In addition to the morpholino moiety, this superiority is attributed to the introduction ethyl 2-cyano-2-(hydroxyimino)acetate, Oxyma in its structure. Oxyma displays high control of optical purity and coupling extension in demanding sequences.
The design of COMU brings together the excellent leaving group ability of Oxyma and the markedly reactive dimethyl morpholino core contained in N-(chloro)morpholino)methylene) -N-methylmethanaminium hexafluorophosphate (DMCH). The advantageous properties of the morpholine moiety observed in benzotriazole aminium salts were also noted in the comparison between HOTU and COMU. Thus, including the dimethylmorpholino skeleton in COMU resulted in 50% higher solubility in DMF than HOTU. In addition, COMU also allowed the preparation of approximately four times more concentrated solutions than benzotriazoles HBTU and HATU in DMF, with direct implications for coupling efficiency. Furthermore, solution phase acylations with COMU are facilitated by the excellent water solubility of its by-products, such as the corresponding urea, resulting in cleaner workup removal in contrast to benzotriazole-based reagents[2]. A distinctive feature of COMU is that its coupling reactions can be monitored by a change of color of the solution, which depends on the base used, thus indicating the endpoint.
Apart from the effect by the Oxyma and the morpholino moieties, X-ray and 13C-NMR studies proved that COMU exists as uronium salt (i.e., O-form). Thus, it belongs to a more reactive subfamily than the aminium salts (N-form) such as HBTU, HATU, HCTU, TBTU, TATU, TCTU, HDMB, HDMA, and HDMC. This structural feature may be partially responsible for the markedly high acylation rate both in solution and solid-phase synthesis. COMU shows a less hazardous safety profile than benzotriazole-based reagents, such as HATU and HBTU, which in addition exhibit unpredictable autocatalytic decompositions and therefore a higher risk of explosion. Furthermore, in contrast to benzotriazole-based reagents, COMU is significantly less likely to cause allergic reaction.
References
[1] Ramon Subiros-Funosas. et al. "Microwave irradiation and COMU: a potent combination for solid-phase peptide synthesis." Tetrahedron Letters 50.11(2009):Tetrahedron Letters.
[2] Ramon Subirós-Funosas. “COMU: scope and limitations of the latest innovation in peptide acyl transfer reagents.” Journal of Peptide Science 19 7 (2013): 408–414.
You may like
Related articles And Qustion
See also
Lastest Price from COMU manufacturers
US $0.00/KG2025-04-21
- CAS:
- 1075198-30-9
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month
US $0.00-0.00/KG2025-04-04
- CAS:
- 1075198-30-9
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton