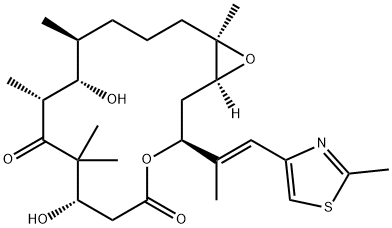
An antineoplastic agent: Epothilone B
Description
Epothilone B (epoB; also called patupilone or EPO 906) is an ideal drug for the treatment of spinal cord injury (SCI), which can reduce scar formation in the lesion site, reactivate the axons' regeneration potential and is convenient for operation[1].
EPO906, or patupilone, is a synthesized version of natural Epothilone B (EpoB) developed by Novartis, with the same structure and, therefore, the same mechanism of action. EPO906 has been part of many preclinical and clinical studies. Due to its conserved activity against taxane-resistant cells, it could be an alternative for patients who relapse after chemotherapy based on paclitaxel and platinum derivatives and present high levels of class III beta-tubulin (TUBB3).
Clinical trial results recognize the potential of patupilone to improve progression-free survival and pain response in castration-resistant prostate cancer and gynecologic cancer, whose relapse events are accompanied by resistance to docetaxel and cisplatin, respectively.
Use
Epothilone B (EpoB) is an antineoplastic agent with FDA approval, which shows the capacity to improve the stability of microtubules and to promote α-tubulin polymerization. EpoB can promote OV-90 and ABT-737 ovarian cancer cells' apoptotic death via respective signaling through the Apo-2L/TRAIL and PI3K/Akt/mTOR pathways. Patupilone promotes neural regeneration after an injury, inducing concerted polymerization of microtubules at the axon tip, which drives axon growth by inhibiting the migration of scar-forming fibroblasts and reactivating neuronal polarization.
EpoB has recently exhibited a regenerative effect on the central nervous system. Systemic administration of subtoxic EpoB doses enhances axonal regeneration and attenuates fibrotic scarring following an injury to the spinal cord by abrogating meningeal fibroblast polarization and migration. Intraperitoneal low-dose EpoB administration has been shown to suppress axonal microtubule deterioration and improve cognitive function in a murine tauopathy model. Furthermore, systemic EpoD administration has been suggested to protect against a murine MPTP-induced parkinsonism model. A recent study found that EpoB alleviates nigrostriatal pathway damage while enhancing motor functionality following intracerebral hemorrhage in mice[2].
Clinical research
The clinical efficacy of patupilone was also evaluated in castration-resistant prostate cancer (mCPRC) patients who had never received chemotherapy. These patients with mCPRC have a poor prognosis, and first-line therapy is mainly based on docetaxel-based regimens, which makes them prone to develop resistance quickly. The treatment based on patupilone + prednisone revealed a better response in view of PSA levels compared to the classical treatment of docetaxel + prednisone in patients who received intravenous (I.V.) patupilone (10 mg/m2 or docetaxel 75 mg/m2 every 3 weeks.
In ovarian cancer, patupilone has been tested clinically with Phase I and II studies. The results showed a modest efficacy profile in patients treated with patupilone, but the treatment did not achieve a significant improvement in overall survival compared to the active control. The most frequent toxicity in the patupilone arm was diarrhea (25.6% grade 3 and 4) and mild peripheral neuropathy (6.2% grade 3 and 4), consistent with Phase I and II trials of monotherapy where diarrhea grade 3 was the most common adverse effect present in 8–29% of patients with the highest incidence in patients with colorectal and prostate cancer, and is the dose-limiting event in therapeutic schemes[3].
In humans, patupilone accumulation in glioblastoma (GBM) tumor tissue is 30 times higher compared to plasma values at 20 min. In this sense, the administration of patupilone before and after surgery in recurrent GBM is safe, improves long-term PFS in patients, and could be an alternative for the treatment of central nervous system (CNS) cancers. However, larger clinical trials are needed to ensure the safety and efficacy of this drug.
References
[1] Liang Mao. “Epothilone B impairs functional recovery after spinal cord injury by increasing secretion of macrophage colony-stimulating factor.” Cell Death & Disease 8 11 (2017): e3162–e3162.
[2] Jianhua Zhou. “Epothilone B Facilitates Peripheral Nerve Regeneration by Promoting Autophagy and Migration in Schwann Cells.” Frontiers in Cellular Neuroscience (2020): 143.
[3] Cecilia Villegas. “Epothilones as Natural Compounds for Novel Anticancer Drugs Development.” International Journal of Molecular Sciences 24 7 (2023).
You may like
See also
Lastest Price from Epothilone B manufacturers

US $5.00-0.50/KG2025-05-29
- CAS:
- 152044-54-7
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $1.00/KG2025-04-21
- CAS:
- 152044-54-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt