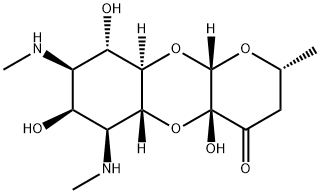
An aminocyclitol compound:Spectinomycin
Spectinomycin is an aminocyclitol compound which has some structural similarities to streptomycin, but it differs by not being an aminoglycoside. Spectinomycin was isolated in 1960 from Streptomyces spectabilis in the Upjohn Research Laboratories (Mason et al., 1961). It was originally known as actinospectacin and was manufactured as the sulfate salt. Later, it was manufactured as the more soluble dihydrochloride salt. The clinical use of this drug has been restricted to the treatment of gonorrhoea.
ANTIMICROBIAL ACTIVITY
Spectinomycin has a wide range of activity against Gram-negative bacteria. Escherichia coli [minimum inhibitory concentration (MIC) 12.5–50 mg/ml], Klebsiella pneumoniae, and the Enterobacter, Salmonella, and Shigella spp. are usually susceptible. Proteus mirabilis and, to a lesser extent, other Proteus spp. are often susceptible. Serratia and Citrobacter spp. are sometimes susceptible, while Providencia spp. and Pseudomonas aeruginosa are always resistant. A high percentage of the Enterobacteriaceae are inhibited by a concentration of r128 mg/l, a concentration which is easily attainable in the urine with normal spectinomycin doses. The greatest activity of spectinomycin is shown against Neisseria gonorrhoeae. Nearly all strains of N. gonorrhoeae in the USA were inhibited by a concentration of 6.3 mg/ml or less in early studies. Some studies indicated that gonococcal strains relatively resistant to penicillin G showed no increased resistance to spectinomycin, but others found that there was a weak correlation between their sensitivities. Gonococcal strains completely resistant to penicillin G without beta-lactamase production were usually susceptible to spectinomycin.
MECHANISM OF DRUG ACTION
Similar to streptomycin, spectinomycin acts at the 30S ribosomal subunit, thereby inhibiting protein synthesis. In E. coli, it was shown to block the swiveling of the head domain of the ribosomal subunit, thereby disrupting the translocation cycle. At high concentrations it is not bactericidal to E. coli. In studies using N. gonorrhoeae, Ward (1977) showed that spectinomycin was more bactericidal than penicillin G, tetracycline, and kanamycin. Spectinomycin also produces alterations in the surface morphology of gonococci, leading to their lysis. This possibly results from the action of spectinomycin on the ribosomes resulting in inhibition of the cytoplasmic membrane proteins and interference with the osmotic integrity of the cell.
PHARMACOKINETICS
Spectinomycin is poorly absorbed after oral administration, but it is well absorbed from i.m. injection sites. A peak serum level of about 100 mg/ml is attained about 1 hour after a 2-g i.m. dose. Doubling the dose nearly doubles the serum concentration. A detectable serum level persists for about 8 hours after a dose. A mean serum level of 64.3 mg/ml was detected at 1 hour in children given an i.m. dose of 40 mg/kg. The drug is little bound to serum proteins.
TOXICITY
Spectinomycin given as a single dose once only appears to be of low toxicity. Willcox (1962) treated 101 patients and observed no side effects. Its low toxicity has been confirmed by others. Occasionally, patients have noted transient dizziness after the injection. A few patients have developed either transient fever, nausea, headache, or moderate discomfort at the injection site. Ototoxicity and nephrotoxicity have not been reported. Rarely, an erythematous rash has Spectinomycin 1031 been noted. When spectinomycin was given in a dose of 2 g four times daily for 21 days to volunteers, no evidence of ototoxicity or nephrotoxicity was detected. Spectinomycin is not cross-allergenic with the penicillins.
Lastest Price from Spectinomycin manufacturers

US $0.00/kg2025-04-25
- CAS:
- 1695-77-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00-0.00/KG2022-05-10
- CAS:
- 1695-77-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20tons