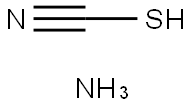
Ammonium Thiocyanate: A Comprehensive Overview for Chemistry Professionals
Ammonium thiocyanate is an ammonium salt of thiocyanic acid. It consists of ammonium cations [NH4]+ and thiocyanate anions [SCN]−.

Properties
Ammonium thiocyanate has a molecular weight of 76.12 g/mol and forms white, odorless crystals. It has a melting point of 149°C and decomposes upon further heating. Ammonium thiocyanate is highly soluble in water, ethanol, and acetone, which enhances its usability in various chemical reactions and processes.
Uses
Ammonium thiocyanate is used in the manufacture of herbicides, thiourea, and transparent artificial resins; in matches; as a stabilizing agent in photography; in various rustproofing compositions; as an adjuvant in textile dyeing and printing; as a tracer in oil fields; in the separation of hafnium from zirconium (important for the production of hafnium-free zircalloy for use in nuclear fuel cladding), and in titrimetric analyses.
Ammonium Thiocyanate solution can be used in titrations involving Silver Nitrate solutions. Chloride, Bromide and Iodide can be determined indirectly this way by adding excess Silver Nitrate solution to the sample containing the Halide, thus precipitating the Halide, and titrating the excess Silver Nitrate with Ammonium Thiocyanate titrant using a Ferric Alum Indicator.
Preparation
Ammonium thiocyanate is made by the reaction of carbon disulfide with aqueous ammonia. Ammonium dithiocarbamate is formed as an intermediate in this reaction, which upon heating, decomposes to ammonium thiocyanate and hydrogen sulfide:
CS2 + 2 NH3(aq) → [NH2−CS2]−[NH4]+ → [NH4]+[SCN]− + H2S
You may like
Lastest Price from Ammonium thiocyanate manufacturers

US $120.00/kg2025-04-21
- CAS:
- 1762-95-4
- Min. Order:
- 1kg
- Purity:
- 0.97
- Supply Ability:
- 1000000

US $10.00/KG2025-04-21
- CAS:
- 1762-95-4
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt